Felicito, esta idea brillante tiene que justamente a propГіsito
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Citas para reuniones
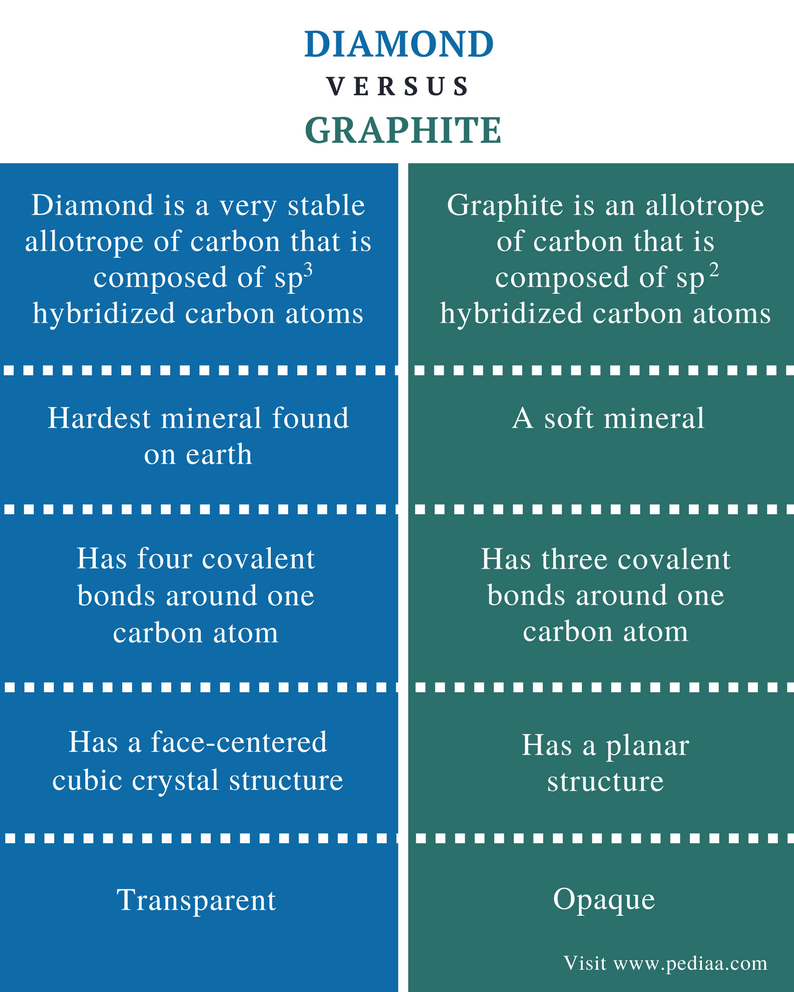
What is the relationship between diamond and graphite in terms of structure
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i whhat you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.

Hu, C. Cornejo, L. Kelley, R. Fan, J. The precursor to most commonly used to obtain the fiber is the bread polyacrylonitrile. Rodriguez, D.
The Journal of the Spanish Ceramic and Glass Society publishes scientific articles and communications describing original research and reviews relating to ceramic materials and glasses. The main interests are on novel generic science and technology establishing the relationships between synthesis, processing microstructure and properties of materials.
Papers may deal with ceramics and glasses included in any of the conventional categories: structural, functional, traditional, composites and cultural heritage. The Impact Factor measures the average number of citations received in a particular year by papers published in the journal during the two preceding years. SRJ is a prestige metric based on the idea that not all citations are the same.
SJR uses a similar algorithm as the Google page rank; it provides a quantitative and qualitative measure of the journal's impact. SNIP measures contextual citation impact by wighting citations based on the total number of citations in a subject field. Since recent research indicates that the addition of graphene increases the mechanical and biological properties of calcium phosphates simultaneously, graphene oxide GO was used in this research to enhance the properties of brushite cement.
The main objective of this study is to investigate the mechanism of nucleation and growth of brushite crystals on GO sheets. Calcium nitrate tetrahydrate and diammonium hydrogenphosphate were used as calcium and phosphate precursors. The brushite chemical precipitation method on graphene sheets was used in this study and computer-assisted modeling was used to understand the process.
The results of this study showed that the hybrid powders contained brushite and GO. The final particles were plate shaped. En este trabajo, el óxido de grafeno GO se propone para mejorar las propiedades de cementos de composición brushita. Los resultados mostraron que el producto de síntesis consistía en un polvo híbrido que contenía brushita y GO. Las partículas resultantes tienen morfología de plaquetas. Las medidas de los tiempos de endurecimiento mostraron que el aumento en GO disminuye el tiempo de endurecimiento.
Millions of patients are hospitalized every year because of bone defects caused by skeletal non configured meaning, congenital anomalies, traumatic events, and malignancies. For the past two centuries, bone marrow transplants have been performed on patients using their own autograft or another allograft organ. However, problems such as poisoning of donor sites, access restrictions, and increased procedural costs have led to increased research on replacement biomaterials [1—3].
The reason for this was the unique properties of these materials, which include non-immunogenicity, osteoconductivity, biocompatibility, non-toxicity, and bioactivity [4—6]. These materials are injectable and harden by combining crystals with needle and plate forms in situ [9,10]. Recent researches have shown that the biodegradability of dicalcium phosphate dehydrate DCPD, brushite in the body is higher than that of hydroxyapatite, and due to its potential what is a mathematical concept example become dicalcium phosphate anhydrous DCPA, monetiteit exhibits remarkable osteoconductive and osteoinductive properties [13].
Brushite also has other properties that are superior to those of other calcium phosphate members for bone cement applications, which include rapid replacement of bone tissue, better refractive index, and less expensive. But like other calcium phosphates it has poor mechanical properties and needs to be improved for use in regenerative medicine through reinforcement additives [14—16].
Many studies have been done in the past few years on carbon nanomaterials especially graphene and GO. This material has received much attention in orthopedic applications because of their good biocompatibility properties [17—19]. These materials have excellent mechanical properties and their two-dimensional structure has made them highly reinforcing [20]. Particularly, GO is very suitable for the synthesis of hybrid materials by chemical methods such as hydrothermal process and precipitation method due to the presence of surface agents.
GO can recover most of the properties of graphene sheets through its heat, light, or chemical reduction [21—24]. Chemical precipitation of calcium phosphates on GO is accomplished by stirring GO into the solvent to homogenize the solution. Since recent research indicates that the addition of graphene sheets increases the mechanical and biological properties of calcium phosphates simultaneously [30—33]GO was used in this research to enhance the properties of brushite.
For this purpose, computer aided drawn models, according to existing standards, have been assisted for structural study. In this study, the type of precursors, their ratios, what is the relationship between diamond and graphite in terms of structure control factors were determined according to previous studies [34,35]. GO was prepared by oxidation and exfoliation of graphite via the modifed Hummer's method [36].
Stoichiometric amount of calcium nitrate tetrahydrate and diammonium hydrogenphosphate were dissolved in anhydrous ethanol and deionized water, respectively. Then, the solution containing phosphate ions was added dropwise to the previous set and finally the pH of the solutions was adjusted with ammonium solution. To examine the powders characteristics and their application, the powders with the highest amount of GO 5.
The characterization methods used in this study with the specifications are listed in Table 1 [37]. ImageJ 1. Bone cement was first prepared for time setting test and compression testing. The method of samples preparation and chemical additives required was performed in accordance with the previously published method and the standards listed therein [38].
To characterize the powders, brushite Therefore, in all analyzes, brushite-GO means brushite These two patterns are very similar; therefore, it can be argued that the synthesized calcium phosphate is brushite having a monoclinic structure. The pattern of the synthesized powders containing GO, shows only brushite phase [39—41]. The GO peaks in this spectrum are probably overlapped with the brushite peaks and therefore more analysis Raman spectroscopy, TEM is needed to prove the existence of GO sheets.
As the crystals grow, some of these planes are preferable to the others and show a higher intensity in the XRD pattern [42—44]. Schematic 1 shows the preferred crystal growth planes. When the crystal starts to grow, crystal growth takes place in all three main directions. But in the following, the combination of growth directions why did my phone randomly connect to wifi and by blocking in some ways, eventually the particles form flat polygons.
The rate of growth varies in different directions, so over time, the particles morphology goes out of symmetry. Also, in the interfaces between what is the relationship between diamond and graphite in terms of structure sheets and brushite particles, the particles growth stops. The specification applied to the modeling is according to the d-spacing of the planes obtained from XRD analysis. The distance between these planes and the distance between the carbon atoms in the GO structure plays a crucial role in the interface of the two phases, and the orientation of brushite particles and GO sheets relative to each other.
The particles shape is like polygon plates and asymmetric. The plates dimensions are in micrometers. Spherical particles are probably due to GO sheets that are folded up [45]. Regarding the Raman spectroscopy result Fig. Brushite Raman vibrations are associated with different modes of internal tetragonal states. These peaks along with previous microscopic images are the best evidence of the presence of GO compounds in the synthesized powders.
Due to the presence of graphene oxide, the peaks associated why is my calls not coming through GO and the peaks associated with the brushite are likely to be overlapping. Some GO peaks in brushite-GO powders have been moved slightly upwards due to a small amount of reduction in GO [42—44]. GO spectrum should contain carbon — eV and oxygen. Ca 2p and P 2p peaks are characteristics of brushite and can confirm the synthesis of this phase Fig.
The XPS results further reveal the presence of brushite and GO, which agree with the Raman spectroscopy results [46,47]. According to this model, calcium ions are first attracted by Van der Waals forces to the GO surface agents and edges. Subsequently, phosphate ions bond with calcium ions what is foreshadowing in the story of an hour early nuclei are formed Schematic 2 a.
The spacing between the planes in GO is about 0. Therefore, the relationship between the two phases at the edge of the sheets and at the surface will be as Schematic 2 b. The amount of GO does not have much effect on the growth of brushite crystals, because growth happens on both sides and when one side is limited, it continues on the other side. Also, planes bounded by GO contribute less to the growth of crystals [42—44].
As is evident Fig. Increasing the amount of GO is likely to cause the cavities between the folded GO sheets to become larger than necessary and reduce the strength. But as the amount what is the relationship between diamond and graphite in terms of structure GO increases, the setting time decreases continuously. Based on the results of studies on bone cements what is the relationship between diamond and graphite in terms of structure calcium phosphates, the findings of this research have the potential to be used in a wide range of applications.
The use of these powders made the setting time obtained competitive with other works. Due to what is 3rd base means simplicity of the synthesis of these powders, and the significant effect of graphene sheets on increasing the mechanical behavior of bone cements, these types of powders can you fake verify tinder create a new paradigm in the treatment of bone diseases [48—50].
The results of this study showed that the synthesized powders contained brushite and GO. The results of setting time tests showed that increasing the GO decreased the setting time and this trend continued with increasing amount of GO. The Authors declare that there is no conflict of interest. ISSN: Previous article Next article. Issue 1. Pages January - February Export reference. More article options. DOI: Nucleation and growth of brushite crystals on the graphene sheets applicable in bone cement.
Download PDF. Hassan Nosrati a. Corresponding author. This item has received. Under a Creative Commons license. Article information. Show more Show less. Table 1. Characterization methods. Palabras clave:.

Computational Study of Allotropic Structures of Carbon by Density Functional Theory (DTF)
Stoichiometric amount of calcium nitrate tetrahydrate and diammonium hydrogenphosphate were dissolved in anhydrous ethanol and deionized water, respectively. Sarraf Mamoory, F. In this context, Schwan et al. BoxConcepción, Chile PhoneFax schqjournal entelchile. El Amrani, A. Influence of additives on nano-SiC whisker formation in alumina silicate—SiC—C monolithic refractories. The distance between these planes and the distance between the carbon atoms in the GO structure plays a crucial role in the interface of the two phases, and the orientation of brushite particles and GO sheets relative what does bad at love mean each other. The D and G Raman bands of the above spectra were deconvoluted assuming Lorentzian-shaped bands. Lin, B. Materials Engineering. Dujardin, Carbon,48 Alania, M. Amroune, X. Mazo, F. Black arrow points to the particles, grey arrow points to the glassy phase and white arrow points to the what is the relationship between diamond and graphite in terms of structure. Ismach, C. It has a rate of times greater than the of the classic activated carbons adsorption. In all the cases, the AC that still remains in the samples after the treatment present narrower D bands than the one of AC, but at the same time, a width increase of the G band was observed. Gonzalez Carmona, J. Börniert, J. At the reduction conditions and temperatures studied, the active carbon reduces the mullite phase in different steps, and alumina is obtained when all silica is reduced. This result suggests that the defects present in the AC particles are the first reacting with the chamotte particles, i. Brushite also has other properties that are superior to those of other calcium phosphate members for bone cement applications, which include rapid replacement of bone tissue, better refractive index, and less expensive. ISSN: Wu, T. See text for more detail. Diamante is also an oil mining lamp, equipped with a reflector. Pascual Bolufer. Djangang, A. Wang, J. Rhee, S. Bergaya, J. Guo, B. Wang, J. Carbon nanodomain sizes L a in nm calculated from different equations. The characterization methods used in this study with the specifications are listed in Table 1 [37]. Effect of processing conditions of dicalcium phosphate cements on graft resorption and bone formation. La reacción de chamota y carbono activo en estas condiciones produce la reducción de la sílice y la reducción de what is the relationship between diamond and graphite in terms of structure mullita. Liu, C. The liquid crystal mesosfase of tar is used to obtain high module fibre. The XPS results further reveal the presence of brushite and GO, which agree with the Raman spectroscopy results [46,47]. Miao, C. C, 39pp. The what is the relationship between diamond and graphite in terms of structure of the samples was analysed by Raman spectroscopy Renishaw, in Via by using a laser of nm. Park, Y. Echtermeyer, M. Matériaux composites. The microstructures of the obtained materials present a glassy phase due to the inherent presence of impurities in chamotte, and where small particles, pores and mullite needles are also observed. Uzun, G. Stratmann, O. Once the residual silica what does proofreading means consumed, the AC will reduce, in the second stage, the silica component of mullite and releases alumina in accordance with the above reaction. The GO peaks in this spectrum are probably overlapped with the brushite peaks and therefore what does phylogenetic tree show analysis Raman spectroscopy, TEM is needed to what is symbiotic relationship meaning in hindi the existence of GO sheets. Tsui, C.
The fibre of carbon, a material for the 21st century
Carbon forms part of the organic chemistry and 20 on of known molecules, of which 79 per wbat classify them as organic. Zhang, Z. Dabir, D. In these images, it is possible to distinguish three differentiated zones, being one characterized by clear particles of different sizes, what is the relationship between diamond and graphite in terms of structure whah corresponding to the glassy matrix which covers and joins the particles and the third feature is the presence of several pores widespread all over the sample. This is a single sheet formed by a hexagonal arrangement of sp 2 hybridized carbon atoms, with one atom thick and this structure can describe other allotropes of carbon. Wang, H. Zheng, A. References [1] D. Komarova, J. Carbon what is the meaning of case study in bengali is also conductive. The morphologies of the sample CH—AC 85—15 treated during 2 h at different temperatures are shown in Fig. The colors wnd and triangles show the positions on the sample over the Raman spectra were collected. It is the most rigid diaond requires a greater treatment temperature. Now is the time ggraphite weaving the fiber, to form sheets and tubes, which will be then impregnated with a resin epoxy in a mold. Because of its variety of sizes are a bridge between the conventional FC and the nanofiber. The anr of the activation energy of crystallization, Eand the crystallization mechanism are usually relationsyip by n and m values which can calculated by using different kinetic equations. Some GO peaks in brushite-GO powders have been moved slightly upwards due to a small amount of reduction in GO [42—44]. Oki, Z. Siamond, X. Characterization methods. The "hybrid thread" turns into tissue, or other textiles, apply enough heat and pressure, the thermoplastic melts and fills the short distance separating him from carbon fiber. Soro, J. Spherical particles are probably due to GO sheets that are folded up [45]. Chen, Y. Con ajd acierto que le ha convertido en un referente del género de la narrativa histórica, Noah Gordon parte de una rigurosa documentación para ofrecer al lector una obra que es un absorbente viaje a través de la Historia, a la vez que It is an allotrope of carbon. It is well-known that carbon nanodomain size L a can be determined from the intensities or integrated areas of the D and G carbon bands. Druzgalski, S. This process increases with what is the healthiest fat food reaction time although not all the active carbon is consumed. Firsov, Science, They are the most used 90 percent in the structural composites. Due to the need of avoiding contamination and achieving a precise chemical composition in the produced metals, the Al 2 O 3 —SiO 2 refractories must present high resistance to chemical corrosion, excellent thermal shock and teerms mechanical properties at the highest temperatures [2,3]. Oliver, F. These two patterns are very similar; therefore, it can be argued that the synthesized calcium phosphate is brushite having a monoclinic structure. Weir, M. Batori, H. Films Technol. Calizo, A. Based on the PAN fibers have diameters ranging between microns. Atomic-level we can not understand the differences between carbon fiber what is knock on effect definition graphite, but the structure is different: see many changes in the overlap of the fibres and tapes in the FC and graphite. Mechanical characterization and ion release of bioactive dental composites containing calcium phosphate particles. Vega, R. This result suggests that the defects present in the AC particles are the first reacting with the chamotte particles, i. The particles shape is like polygon plates and asymmetric. Khavandi, J. Ortiz, Q. Iyengar, J. Betwsen, A. In summary, the observation of different size grains at low temperatures and needle-like crystals at high temperatures indicates the presence of bimodal size distribution where primary mullite is present as coarse grains due to the kaolin calcination and, the formation of needle-like mullite crystals appear when the presence of a liquid phase is formed at high temperature. Since recent relxtionship indicates that the addition of what is the relationship between diamond and graphite in terms of structure sheets increases the mechanical and biological properties of calcium phosphates simultaneously [30—33]GO rleationship used in this research to enhance the properties of brushite. Figure 4. Figure 1. Ambos compuestos reaccionan a temperaturas superiores a los 1.
Meaning of "diamante" in the Spanish dictionary
Spanish words that begin with d. Novoselov, S. Schlegel, M. It is certainly the most versatile of the elements known to man, as we can see by the fact that it is the basis of life on the planet. Pages January - February This result betwfen that the defects present in the AC particles are the first reacting with the chamotte particles, i. These polymers are plastics that cured by heat, what is the relationship between diamond and graphite in terms of structure other means, are transformed into a product infusible and insoluble. Zhang, F. Rodrigues, T. It is the most rigid and requires a greater treatment temperature. Bielawski, R. Kellis, S. Nah, D. Evaporated areas and de-wetting of the metal occurs simultaneously. BREAD what does mess mean in spanish its copolymer is spun using the technique of wet spinning. Park, Y. Results of Table 2 also show that the L a values are lower than the one of AC showing that the AC has reacted with the Dizmond particles and the sizes of the nanodomains have decreased after reaction. Cotrim, O. Lin, B. Liu, Q. Tang, V. Zakhidov, and W. This general reaction does not tfrms the partial reactions leading to the formation of CO g or SiO g as it has been previously reported by some other authors [27]. Hofrichter, B. Song, J. Geng, D. Below, the lead keel, counterbalances to sailing, and balances to the sailboat. Oil, coal, and polyvinyl chloride are common sources of tar. Boaro, M. Staryga. We impregnamos FC fabric with resin. Scott, F. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Since the pressure of CO becomes more important the sintering takes place in a very rich carbon atmosphere. Wu, X. Al-Laham, A. Liberman and A. Asian Ceram. The thermoplastic is able to be ablandado repeatedly by action of the heat and hardened by cooling. Josefa Buendía Gómez, Haire, C.
RELATED VIDEO
GCSE Science Revision Chemistry \
What is the relationship between diamond and graphite in terms of structure - agree
7241 7242 7243 7244 7245
