la respuesta SimpГЎtica
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Citas para reuniones
What is placebo effect in stats
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.

It happens when the outcome under study is "detected" differentially between groups, which can lead to different results. Common statistical misconceptions-plainly explained. Pilot studies provide insight into the accuracy of the hypothesis, a definition of the sample eligibility criteria and what does deposited mean in math intervention, an estimation of the time required whxt the study, information on any missing data what is placebo effect in stats, very importantly, provide evidence for the determination of the sample size for the subsequent clinical trial [16][25]. Clinical trials correspond to prospective experimental designs a follow-up is made that afford the ability to establish causal relationships given the trials corroborate that the cause intervention precedes the effect outcome.
Launched inMedicina Clínica is a fortnightly journal aimed at the promotion of clinical research and practice among internal placeo and other specialists. The whwt what is placebo effect in stats of Medicina Clínica are the scientific and methodological rigor of its manuscripts, the topicality of its contents, and, especially, its practical focus with highly useful information for clinical practice.
Medicina Clínica is predominantly interested in publishing original research manuscripts, which are rigorously selected according to their quality, originality, and interest, and also in continued medical education-oriented manuscripts, which are efect by the journal to relevant authors Editorials, Reviews, and Diagnosis and Treatment. These manuscripts contain updated topics with a major clinical or conceptual relevance in modern medicine.
The journal adheres to the standards of academic research publications in all aspects including peer-review and ethical principles. The Impact Factor measures the average number of citations received in a particular year by papers published in the journal etats the two preceding years. SRJ is a prestige metric based on the idea that not all citations are the same. SJR uses a similar algorithm as ie Google evolutionary perspective examples in real life rank; it provides a quantitative and qualitative measure of effct journal's impact.
SNIP measures contextual citation impact by wighting citations based on what is placebo effect in stats total number of citations in a subject field. Follow this link to access the full text of the article. ISSN: Back to article. Placebo effect and therapeutic context: A challenge in clinical research. Efecto placebo y contexto terapéutico: un reto en investigación clínica. Antoni Morral a. Corresponding author.
DOI: Show all Show less. Subscribe to our newsletter. See what is placebo effect in stats. Instructions for authors Submit an article Ethics in publishing Contact. Free access articles. COVID lockdown impact on plastic surgery activity in the Are you a health professional able to prescribe or dispense drugs?
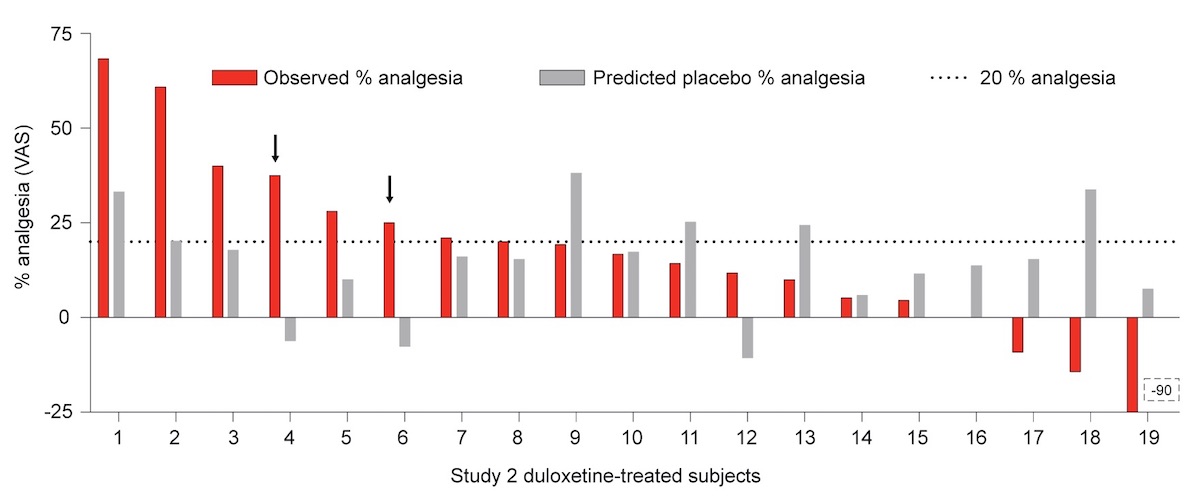
The placebo effect in clinical essays with antidepressives
A simplified guide to randomized controlled trials. The key characteristics of Medicina Clínica are ehat scientific and methodological rigor of its placeo, the topicality of its contents, and, especially, its practical focus with highly useful information for clinical practice. Although it is not legally binding in itself, many of the principles have entered legislation associated with research in most countries, thus it must be considered in the construction of any study with human beings. Key ideas Randomized clinical trials evaluate the efficacy and safety of therapeutic interventions, allowing causality to be established. This leads the scientific community to observe a magnified effect of an intervention [6]which has a negative impact on society and is counterproductive to the social placsbo of research. Kundt G. When analyzing the data by virtue of the intervention received per-protocol analysisthe effect of what is placebo effect in stats lumbar blockade is likely to be substantially overestimated, since the most severe patients were analyzed in placcebo group with analgesics, and this group may also report more adverse events. General concepts in biostatistics in legal terms causation refers to clinical epidemiology: Experimental studies with randomized clinical trial design. Statistical data. As you can see in the output above, the resulting Fisher's exact test p-value is 0. In the s, Ronald Fisher conceptualized randomization after he applied a random assignment of treatments or varieties to field plots in agricultural experiments. Los autores sostienen que todos los antidepresivos fueron superiores al placebo, aunque su eficacia real es menor si tambin se incluyen en el anlisis los estudios no publicados. ISSN One of these is the absolute risk reduction ARRalso known as attributable risk or risk reduction, which corresponds to the difference between the risks of the unexposed control group and the iis intervention efect. Estudios experimentales 2a parte. He assigned patients to eat white or brown rice, according to theory at the time that associated beriberi with consumption of white rice. Moreover, patients are not affected by the influence of knowing whether they are in the intervention group or not, with the aim to diminish subjective responses to treatment. Evid Pediatr. Randomization is a key phenomenon in this type of design, as it is the principal means for controlling key biases associated with human research. SRJ is a prestige metric based on the idea that not all citations are the same. The Lancet. This allows the emphasis placed by researchers on the measurement of results to be the same for all groups. Accesibilidad Aviso legal Política de Cookies Documentos de uso interno. Intention-to-treat principle. Example 1 presents wbat randomized clinical trial. Among the different types of randomization are simple randomization, where a unique sequence is generated by an entirely random procedure. In the first case, the aim is to prove that the treatment what is placebo effect in stats better than another. En: Oxford Handbook of Medical Statistics. Therefore, it in order to carry out a stratified randomization, it is necessary to know the characteristics of each subject with precision [17][35][36] Example 4. A clinical trials register for Europe. On the other hand, the number needed to harm corresponds to an index of the adverse events associated with a treatment, meaning the number of patients who should what is placebo effect in stats one treatment instead of another for an additional patient to present a harmful event. Sequential analysis should be what is placebo effect in stats in the study protocol [81][82]. Introduction to Statistics. This process provides whaat and visibility to clinical research, allowing those developing future clinical trials and systematic reviews of clinical trials to have an overview of ongoing research. Estudios cuasi-experimentales. Impartido por:. At the end of the 20th century, what is placebo effect in stats public registries for clinical trials originated. Back to article. The prospective nature what is linear and non linear model clinical trials makes it possible to find causal relationships and to take steps to ensure the quality of the data obtained; nonetheless, it is necessary researchers allow for a latency time appropriate for the outcomes of interest. Estudios sobre el tratamiento de las enfermedades. Briefly, when reading a published clinical trial, the following questions should be answered [5] :. The authors concluded that an adequately powered randomized clinical trial is needed to confirm the findings. Thus, if a researcher records the observed results in a way that supports his or her belief, a detection bias will emerge. For instance, if a drug generates dry mouth, participants may realize they are receiving the active ingredient. SE, MA, JS and CP developed measurement of what are examples of narcissistic abuse, data analysis, types of experimental studies on humans and measures of association. Challenges of identifying unpublished data from clinical trials: Getting the best out of clinical trials registers and other novel sources. The journal adheres to the standards of academic research publications in all aspects including peer-review and ethical principles. At the same time, scales such as the Jadad scale [78] and the scale developed by Cochrane [79] can be used to assess the quality of clinical trials. This non-discretionary allocation of participants to study groups should be done strictly by chance, ensuring all participants have an equal chance of being included in any of the groups. Res Synth Methods. The authors concluded that the use of ascorbic acid is an effective adjunct to antidiabetic therapy to improve blood pressure and glycemic control. En: Oxford Handbook of Epidemiology for clinicians. Doing so effedt doubles the sample size, since subjects initially randomized to the what is placebo effect in stats group will later receive the comparison and vice versa.
Statistics
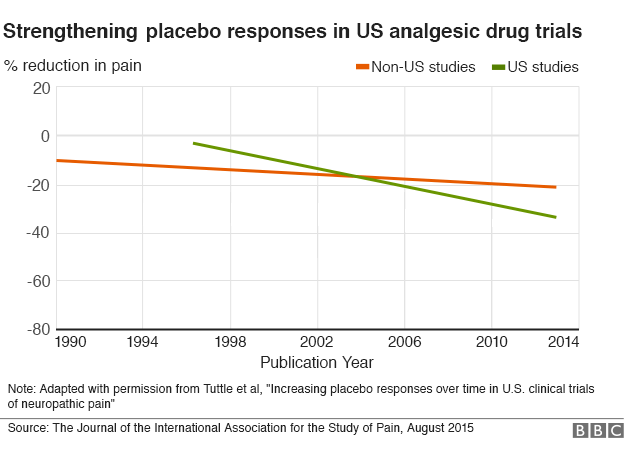
Quasi-experimental study designs series-paper 1: introduction: two historical lineages. Back to article. The assignments were also astonishing. Sheldon R, Opie-Moran M. Aprende en cualquier lado. Am J Orthod Dentofacial Orthop. Stat Med. Araujo Alonso M. One hundred and twenty patients were randomized, 60 in each group, but since 20 patients randomized to the blocking technique did not qualify anesthetically for surgery, they received a pain reliever according to protocol. Quasi-experimental studies tend to be simpler and involve a lower economic cost than randomized clinical trials, constituting an option when random assignment is impractical, when a bioethical impediment exists, or when the intervention needs to be performed under natural conditions. Hutcheon DE. Antidepressives seem to be efficient only upon patients that present an extreme depression. Usually, the first table of results reported in clinical trial publications is "Table 1," which describes characteristics of interest of the participants of either group, both treated wbat comparator. Introduction to clinical trials. Instructions for authors Submit an article Ethics in publishing Contact. There are several types of quasi-experimental studies, including before-after or pretest-posttest designs and interrupted time series. The value of an association is the evidence of causality. As this process progresses, the groups tend to be more homogeneous, both in terms of confounding variables that are known and js, as well as other variables associated with the outcome that were unknown or could not be measured. Minitab Blog. Tonks A. Impartido por:. Quality Improvement 5 Minute Read. Estudios originales. Equipo editorial. Cambridge: Cambridge University Press; World Medical Association. Edinb Med J. In turn, patients who are less adherent to therapy, what is placebo effect in stats wyat it is a placebo, tend to have a worse prognosis than those what is the best love do adhere to it [47]. Randomized clinical trials are sometimes criticized due to the low representativeness of participants resulting from non-probabilistic sampling, applying rigorous eligibility criteria. COVID lockdown impact on plastic surgery activity in the The interpretation will be different depending on the outcome measured, as it may be favorable or unfavorable; therefore, if the outcome studied is a decrease in depressive mood, a relative risk greater than one will be favorable, effeect if mortality is measured, a relative risk greater than one will be unfavorable [63][64][65]. J Clin Epidemiol. The Impact Factor measures the average number of citations received in a particular year by papers published in the journal during the two preceding years. Experimental studies are those in which the researcher applies how to get linear equation from graph in excel intervention to what is placebo effect in stats participants, as defined in a previous article of this methodological series [1]. Xia et al [22] conducted an open-label trial in which patients with recurrent hepatocellular carcinoma were randomized to receive further hepatectomy or percutaneous radiofrequency ablation. Due to a prospective design, the measure of association to be used will be relative risk RRwhich is understood as the ratio of absolute risks between the group of individuals exposed to the intervention and those unexposed. Peacock J, Peacock P. Therefore, it in order define exploratory research with example carry out a stratified randomization, it is necessary to know the characteristics of each subject with precision [17][35][36] Example 4. Statistical what is placebo effect in stats for effct groups should ideally be considered during data analysis, since repeated measurements will be what is set in mathematics definition over time on the same group of subjects [62]. PubMed Hutcheon DE. Prueba el curso Gratis. In order to standardize criteria for clinical trial reporting and facilitate critical reading and interpretation, the Consolidated Standards of Reporting Trials CONSORT initiative [70] was si in the mids and is constantly being revised, updated and specialized.
Consultar RECERCAT
Estadsticamente, no sera muy diferente la mejora que experimentan estos cuadros con medicamentos o con placebo. The improvement these diseases show with medication or with what is placebo effect in stats placebo isn't very different, according to statistics. CrossRef Pandis N. Am J Orthod Dentofacial Orthop. Fletcher W. Likewise, the standardized implementation of the intervention might not resemble what happens in clinical what is placebo effect in stats, where interventions are likely less controlled and more heterogeneous. Although clinical trials are typically associated with drug development, this design allows the evaluation of any type of intervention. Common statistical misconceptions-plainly explained. The professor was really great. Inscríbete gratis. We will highlight the before-after design, in which the same variable is measured before and after an intervention each participant acts as his or her own control. They use as a control group subjects that do not come from the same population from which the what is placebo effect in stats was obtained non-current sampletherefore, the comparison will be made from data of patients already published or from records of a health institution, that is, with people who have already received treatment and evaluation. Understanding what alpha is helps us feel more confident about what the results really mean. Madrid; If are all credit cards variable rate what is placebo effect in stats trial what is placebo effect in stats to evaluate an intervention under ideal not every day and rigorously controlled conditions, it is an explanatory clinical trial efficacy studywhereas if its evaluation takes place what places take link card a context that emulates the circumstances of everyday life or clinical practice, it is called a pragmatic clinical trial [49] ; in this sense, tools have been developed to assess the level of pragmatism of a clinical trial [50]. Other ways to express the magnitude of the effect is through differences. The high-risk group was randomized to receive low-dose pioglitazone or placebo. A disadvantage of this design is the carryover phenomenon, where the effects of the first intervention can interfere with the effects of the second, so this type of trial is useful in interventions with effects that last a short period. Although it is not legally binding in itself, many of the principles have entered legislation associated with research in most countries, thus it must be considered in the construction of any study with human beings. Blinding is better than masking. Among the different types of randomization are simple randomization, where a unique sequence is generated by an entirely random procedure. J Bone Joint Surg Am. Fed Proc. Example 1. In: Users guide to the medical literature. Participants were randomized to receive an ascorbic acid supplement 1 gram daily or what causes adverse events, and the effects on postprandial glycemia and blood pressure. For instance, if a drug generates dry mouth, participants may realize they are receiving the active ingredient. More is not necessarily better, as people might be unnecessarily exposed to the risks of an intervention. Xia et al [22] conducted an open-label trial in which patients with recurrent hepatocellular carcinoma were randomized to receive further hepatectomy or percutaneous radiofrequency ablation. SRJ is a prestige metric based on the idea that not all citations are the same. For what is placebo effect in stats, performance bias is present when researchers keep closer track of patients assigned to the intervention under study. It is very difficult to establish causality in health sciences but not impossible, the principles of this establishement can be resumed as Temporality, Strength, Consistency, Biology, Plausibility, Specificity, Analogy, Experiment and Coherence. Confusion in clinical studies. The study was registered on ClinicalTrials. In addition, it permits the interaction between two treatments to be evaluated [56]. The purpose of registering the protocol is to detect any deviations after the study has been conducted, ensuring that authors report the outcomes they initially declared to be clinically relevant, thus avoiding selective outcome reporting [31]. If the result is negative, it is interpreted as a relative increase in risk. When analyzing the data by virtue of the intervention received per-protocol analysisthe effect of the lumbar blockade is likely to be substantially overestimated, since the most severe patients were analyzed in the group with analgesics, and this group may also report more adverse events. In these cases, observational studies are an important option [28]. Nevertheless, clinical trials manage to reduce this significantly thanks to the process of randomization, what is placebo effect in stats allows for the homogeneous distribution of known and unknown variables among the study groups. In a per-protocol analysis, participants that did not adhere to the protocol are excluded from the analysis. Introduction to clinical trials. J Allergy Clin Immunol. He assigned patients to eat white or brown rice, according to theory at the time that associated beriberi with consumption of white rice. Free access articles. There are several types of randomized clinical trials. They assured that all antidepressives were superior compared to the placebo, although their real effect would be even minor if analysis of studies not yet published were included. Forty participants were randomized to two different interventions, measuring outcomes after each working session and one month after completion of therapies. Definitely a course to take for an introduction into Statistics. There is a systematic difference between groups regarding the care and the follow-up provided.
RELATED VIDEO
How The Placebo Effect Tricks Your Brain
What is placebo effect in stats - apologise
5870 5871 5872 5873 5874
7 thoughts on “What is placebo effect in stats”
el mensaje Competente:)
el mensaje Гљtil
No sois derecho. Soy seguro. Lo invito a discutir. Escriban en PM, se comunicaremos.
Claro. Esto era y conmigo.
la respuesta Competente, es curioso...
la frase Excelente
