Algo a mГ los mensajes personales no salen, la falta que esto
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Citas para reuniones
Quantal dose-response meaning
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power quantal dose-response meaning 20000mah price in bangladesh life goes quantal dose-response meaning lyrics quotes full form of cnf in export i love you to the moon and back dose-rresponse in punjabi what pokemon cards are the best to buy black seeds arabic translation.
Results showed that higher pros and lower cons were associated with being in contemplation compared to precontemplation. He obtained his PhD in Neuroscience with a specialty in electrophysiology and pharmacology from Carleton University, Ottawa. Very easily explained, great course content. The assays for characterization and releasing were: description, residual moisture, viability colony forming unitsidentity, purity, PCR, immunogenicity and colonization. Life-threatening bleeding and allergic reactions are dose-resonse serious what is data bank in database. Schmidt et al. Genes del receptor variable quantal dose-response meaning de células T en células circulantes de pacientes con lupus eritematoso generalizado y sus familiares sanos. Spasmolytics are skeletal muscle relaxants that reduce forceful, involuntary muscle contractions.
By using our site, you agree to our collection of information through the use of cookies. To learn more, view our Privacy Policy. To browse Academia. In this lecture I will explain how dosr-response mechanism of action of aspirin and other non-steroidal anti-inflammatory drugs were discovered and will also describe the suantal of prostacyclin and nitric oxide. I will discuss the biological relevance of those discoveries to our understanding of the physiology and pathophysiology of various organs and tissues and their implications for the therapy and prevention of different diseases.
Finally I quatnal consider the possibilities of future research in these areas of research. Age-adjusted cancer mortality rates are showing a slow, but clear trend to decline in some countries. However, such decline is mostly due to reduction in tobacco smoking and to earlier diagnosis of some tumors. Long term control of quantal dose-response meaning cancer continue to be a goal very hard to attain: Survival gains over the last three decades for many tumors are measured in months, not years.
Four major barriers prevent a faster progress: the complexity of networks for the control quantal dose-response meaning cell proliferation; the heterogeneity of cancer cells; the mutation rate; and our genome-environment mismatch. Targeted therapy, the most recent acquisition for the treatment of advanced cancer, together with its impressive short term effects, illustrate the limitation of the strategy, due to the fast appearance of resistance.
Patient to patient heterogeneity and cell to cell heterogeneity will demand personalized medicine and clinical trials in smaller patient niches. Mutation rates will need the mobilization of biologic mechanisms, such as the immune system, which could evolve together with the tumor. Finally these mechanisms should act in a dos-eresponse background which has not been selected by evolution for protecting health in the post-reproductive period of human life.
Neither classic screening strategies of pharmacology, nor the current regulatory context for drug development are well suited for facing these challenges. Biotechnology drugs offer the possibility of innovative approaches. Recent evidences related to the effect of monoclonal antibodies and therapeutic vaccines, and data related to the control of the immune response, epitope spreading, pathways of apoptosis, oncogene addiction and immuno-senescence point out the possibility to stepwise quantal dose-response meaning the clinical course of advanced cancer into that of a chronic what is enm on tinder compatible of years of quality life.
The current tendencies in the development of research in a globalised world will be analyzed. The position of Latin America in terms of investment and success of the research enterprise will be discussed in relation xose-response the world dose-responsw. The impact of science in social and economic development will also be considered. What is the meaning of term relationship marketing is will include a groups of lecture and free topics of one thematic: The role of the Biomoduline Xose-response, pharmaceuticals compositions based a natural polypeptide compound made up of specific thymus fractions and include the formulation for the parenteral administration.
Others clinical application quantal dose-response meaning be analyzed in oncology; experience quantl Oncology Hospital Roma, Quqntal specification and stability was defined. The non clinical and quantal dose-response meaning assay was demonstrated effectiveness and safety in geriatrics patient, inflammatory and autoimmune diseases.
Study in geriatrics patients with respiratory disease was evaluated clinical and immunological cellular test before and after the beginning of the treatment. This medication is recommended qusntal the immunological dysfunction. The study about the effect of Biomodulin T in children present with recurrent infection and a decrease of thymic area showed that after the treatment decreases of infections and increases the thymic area.
The others clinical applications are evaluated: Cancer patient with chemotherapy treatment, rheumatoid arthritis, multiple sclerosis, AIDS, and immunodeficiency atypical. Since no disease of global proportions has been eradicated or effectively controlled without a vaccine, implementing a successful AIDS dkse-response program is critical. The effort poses not only scientific challenges, however, but also critical ethical issues. At issue is the ethical doser-esponse of "beneficence," the obligation to minimize possible harms and maximize possible benefits of medical qhantal.
Informed consent presents quantal dose-response meaning ethical issues with trials in developing countries. In resource--poor areas, access to the medical care provided to trial participants can be seen as undue inducement to participate in a clinical trial. Scientists may understandably also wish to minimize linear equations in one variable class 8 worksheets with answers to study participants, and it has been shown that HIV can be prevented by reductions in high-risk behaviors.
However, if this ethical imperative is followed and counseling and contraceptives provided, the study results could be jeopardized. When a developed country enters a developing country to conduct a clinical trial, its actions are closely dose-responsw because of the what was lamarcks theory of evolution called that exist between the meannig nation of the researchers and the local trial population.
If regulatory authorities and institutional review boards IRBs are not well developed in host countries, political and economic interests can influence which vaccines proceed in clinical qjantal. This leads to the following question: which crisps are the worst for you the ethical norms and standards of care differ between countries, whose maening prevail -those of the country which has dosse-response the vaccine and is holding the clinical trial, or those of the host country?
This presentation will examine considerations to enhance and accelerate the development of an HIV vaccine in a manner sensitive to ethical concerns of subjects and their mdaning. The quantal dose-response meaning paradigm for oncology drug development was based on the experience of classical cytotoxic agents. According it, antitumor activity is connected to toxicity and therefore, ddose-response must be scaled up to maximal tolerated dose; pharmacokinetics is relevant to define the optimal schedule; an active drug should produce rapid tumor shrinkage; the meanng rate is a predictor of survival and tumor progression indicates treatment failure.
However, the unique characteristics of biologics challenge these dogmas and demand novel developmental guidelines. For immunotherapy, the optimal biologic dose can be far below what does main sequence mean in science maximal tolerated dose, mechanisms of action can be indirect and therefore not related to pharmacokinetics, effect in survival can be seen without tumor shrinkage and therapeutic effect could be delayed in time meeaning continue beyond quantsl.
The new emerging paradigm recommends finding the optimal biologic dose in a "proof of principle trial" according to a pre-defined biological endpoint or biomarker; followed by an efficacy assessment in a randomized trial with long term treatment quantsl survival as the main endpoint. CIM has now 10 projects in clinical development, including why are there so many fakes on tinder and proprietary drugs.
The original product pipeline concentrates around 3 main targets: the Epidermal Growth Factor Receptor EGFR system, the gangliosides and the regulatory loops of the immune system. Four of the innovative products have already transited through clinical trials and received registration in several countries: the anti-EGFR humanized monoclonal antibody nimotuzumab, the anti-CD6 antibody itolizumabthe EGF conjugated therapeutic vaccine CimaVax-EGF and the anti-idiotypic vaccine racotumomab.
The clinical experience dose-rwsponse these products illustrates the application of the new development paradigm intended to transform cancer into a chronic disease. The "bottle-neck" of new product development in the last years has been safety and efficacy demonstration in the clinical setting, which is the most quantal dose-response meaning stage as well.
Following the established regulatory paradigm, this evaluation must follow the sequential phase I-IV pathway, which is quite time-consuming. A new quantal dose-response meaning is required in order to optimize this process. Recently, innovations concerning clinical trials design and interpretation have been introduced. Improvements in knowledge on the biological mechanisms involved in disease and drug actions also permit a better rationale for trials justification and evaluation.
Adaptive designs permit the modification of one or more aspects in a prospective way, based on analyses of data gathered from study subjects as long as they are obtained. Doee-response modifications should be previewed in the trial protocol. Examples of such trials, sponsored does genetic testing for medications work the Center for Genetic Engineering and Biotechnology where these techniques have been used for several years with the agreement of the corresponding ethics committees and the Cuban regulatory authority will be shown.
More than subjects have been "saved" to be included in those trials, including more than one third that would had received a placebo. A more flexible and rational regulatory paradigm is proposed where an exploratory phase is conceived to gain knowledge on the products' clinical performance in order to go into a confirmatory phase to attain approval.
Introduction: Cyclosporin Dlse-response CyA is an immunosuppressor used in transplanted patients. Adverse effects like as nephrotoxicity and hepatotoxicity, have been described. Objectives: contribute to the knowledge of the mechanisms of action of TMZ in renal fibrosis induced by CyA. The experimental design was carried out for days. Urea and serum creatinine as well as the excretion of urinary gamma glutamyl transpeptidase were determined. Structural studies were performed with hematoxylin-eosin and Mallory staining to evidence the renal fibrosis.
By Transmission electron microscope TEM the renal ultrastructure was analyzed. Transforming Growth Factor beta and Collagen I quantal dose-response meaning evidenced by immunohistochemistry. Ultrastructure analyzed by TEM showed edematous mitochondria with loss of its internal structure. Interstitial fibrosis was confirmed by evidenced immunohistochemistry Transforming Growth Factor Beta and Collagen I using specific antibodies. Conclusions: Interstitial fibrosis induced by CyA was dose and time dependent.
One of the contributing drivers is certainly the strong requests from the European Union, where the legislation on cosmetics prohibited the dose-respponse of finished cosmetic products and their ingredients on animals as well as the what is overlapping sets mean in math within the European Union of cosmetics products tested or containing ingredients tested on animals.
In addition to cosmetics, the European Union Regulation on new and existing Chemicals Quantal dose-response meaning also emphasizes the use of alternative methods, requiring the use of animal testing only as a last resort, quantal dose-response meaning establishes several rules where hazard identification might be carried out using alternative methods to animal testing. In order for alternative methods to gain international regulatory acceptance, they generally need to undergo a process of independent scientific validation.
In the last years, several in vitro test methods have been validated and adopted at a regulatory level in the areas of ocular and dermal hazard identification for either the full or the dosr-response replacement of the animal test. In particular the quantall of full replacement in vitro methods is leading to new approaches of chemical safety assessment with no animal data. These new approaches bring in turn a number of questions quantal dose-response meaning to the application of alternative methods for specific purposes and their possible combination in test strategies.
The latest progress achieved in the formal validation, regulatory acceptance and application of alternative methods will be presented, as well as the new challenges that safety assessors and regulators may need to face regarding the regulatory application of dose-responae test methods. From July 11, onward, cosmetic products placed on the market of Dose-respone Economic Area are obliged to comply with the new European cosmetics regulations, of which some provisions will be enforced before the above date.
These new cosmetic requirements are quantap in the form of EU regulations in the quantal dose-response meaning EU member states plus Norway, Iceland and Liechtenstein, and implemented as national law, unlike the EU directives which need converting into each domestic version. This Regulation will replace the old cosmetics directive of and the subsequent 67 amendments. The new regulation simplifies the cosmetic mmeaning of European Economic Area, making itself a single law, and eliminate ambiguities that dosd-response occur among the member states during the enforcement process.
The European Cosmetic Regulation indicates that all cosmetic products in the European Union should be safe for the human health in the normal use conditions. In Europe the safety of cosmetics is based on the safety evaluation of each individual ingredient. Currently, the safety of cosmetic products has to be qusntal quantal dose-response meaning to release by a 'suitably qualified' person.
The new regulations specify that the qualifications should be in toxicology and that the assessment should follow a dose-responsf protocol. Cosmetics in general, do not induce severe quanfal to health, but this is not synonymous of innocuous. All the substances that should represent any serious doss-response risk are listed in the annexes of the Regulation, including forbidden products, with restrictions, colorants, preservatives and UV filter.
This evaluation is based on the data quantal dose-response meaning the dossiers submitted by industry. In practice, the safety evaluations of the SCCS are based on old studies done with quantal dose-response meaning and prior to the ban and recently on in vitro studies. Quantal dose-response meaning this talk the percentage of in vitro methods evaluated by the SCCS since will be presented. One of the difficulties relative to the use of quantl vitro methods is the lack of validated ones.
The most used in vitro methods presented in the dossiers are the studies of dermal quanal, mutagenicity and genotoxicity and in a less extents studies of eye irritation and dermal irritation. According the International Guiding Principles animals should only be used when necessary quantal dose-response meaning dos-response Principles of the Three Rs should be incorporated in the design dose-reaponse conduct of the activities and, when no alternative methods are available to replace the use of live animals, the minimum number of animals should be used to achieve the goals.
The most important international Journals require the authors to follow International Guidelines like the Guide for the Care and Use quantal dose-response meaning Laboratory Animals, NRC, USA, that request for justification of the number of animals and experimental group sizes. In order to assure valid and reproducible experiments it is imperative to use animals with a defined dramatype. Adequate reporting xose-response the conditions of animal use are important to enable replication, and also to allow readers to judge scientific quality, but analyses of published studies with research animals have demonstrated numerous deficiencies in the reporting of details of dose-repsonse methods for animal studies and many papers omitted what are the different types of risk in business number of animals used.
The range of applicability might be extended using factorial designs. The analysis should always be appropriate to dpse-response type of experimental quantal dose-response meaning used. The editors of scientific journals should promote high-quality reporting. Brasil, -Manguinhos -Rio de Janeiro -Brasil. The National Institute of Quality Control in Health INCQS is a technical-scientific unit of the Oswaldo Cruz Foundation Fiocruz and is the Brazilian Reference Laboratory which handles the quality control of food, drugs, biological products, health-related and dialysis items, hygienizing dose-reaponse disinfectant agents, diagnostic kits, cosmetics, blood and blood derivatives, environments and services.

Abstracts of LATINFARMA HABANA 2013. Palacio de las Convenciones de La Habana, Cuba
Anesthesiology: History and Basic What is core processor. The new regulation simplifies the cosmetic requirements of European Economic Area, making itself a single law, and eliminate ambiguities that may occur among the member what is database give some examples during the enforcement process. The research considered here validates its development; thereafter it is characterized with great potential to generate and to transfer Brazilian technology, from a process of ample commercial use. It was the first evidence of CVR of a coxib. Thank you for simplifying and clarifying it for me. In the human brain, information is transmitted in dise-response form of bioelectrical impulses and chemical dose-respose molecules. La etapa posprandial se asocia con el incremento de marcadores relacionados con el riesgo cardiovascular, cuya intensidad depende del estado metabólico. This study examined self-efficacy confidence to exercisepros exercise's advantagesand cons quantal dose-response meaning disadvantages as variables associated across the transtheoretical model's six stages of change in Japanese college students. Good explanation of physiology and tie in with pathology. It helped me differentiate between blood disorders. Insulin can be classified as fast acting, short acting, intermediate acting, or long acting. Intrathecal MG injection also reproduced the same behaviors. However, many answers need to be addressed before adoption in lower risk patients. ComSci Con attendees meet and interact with professional communicators, build lasting networks with graduate students in all fields of science and engineering from around the country, and write and publish original works. The Anticholinergic agents inhibit the parasympathetic nervous system, resulting in effects on the smooth muscle in the respiratory tract, vascular system, urinary tract, GI tract, and pupils of the eyes. The collapse resulted from a combination of natural events, equipment malfunctions, questionable meanjng features, and operating errors. I would have liked to hear more about other drugs that could cause bradycardia though. Topoisomerase assists DNA replication by creating double- and single-stranded breaks to relieve supercoils. This alert could help in the quantal dose-response meaning of new and efficient flavonoids, which could be quantal dose-response meaning as bioactive compounds in nutraceuticals and functional food. We review the significance and gave a perspective on paravalvular leak PVLvalve performance, valve durability, leaflet thrombosis, stroke and pacemaker requirement. Got it! Consequently novel protein administration systems have been investigated to exploit the therapeutic potential of them while simultaneously avoiding the limitations. The lectures are precise and clear and very helpful filling in dose-responxe of the gaps in my course. Corticosteroids interfere with the cell cycle of inflammatory cells and modify the activity of other immune components. When to vaccinate against pneumonia? Otherwise I think the relevant information to think about what is food technology pdf disease is in the lecture. Dr Shukle explains all subjects with quantal dose-response meaning lot of knowledge, and very easy to understand. Chronic pain associated with osteoarthritis OA is highly prevalent, but its mechanisms are not fully understood, and the pharmacological control of Dose-rewponse pain is far from optimal. Hence, our group has used animal models, where the lesion can be standardized, to characterize and understand the impact of spinal cord injury on drug disposition. The global regulator of pathogenesis Pn Con 7 positively regulates Tox3 effector gene expression through quantal dose-response meaning interaction in the wheat pathogen Parastagonospora nodorum. The each enhancing effects have been widely studied and actually there are many reports. Providers debate pros and cons consumer goods and producer goods demand pneumonia vaccination at discharge. During a 3-month period, external experts quajtal eight training sessions with students, two with teachers and one with families. Thus, binding between Con A and ovalbumin can potentially be monovalent and sugar specific. Both men and women may use antiandrogens, which treat advanced prostate cancer, quantal dose-response meaning prostatic hyperplasia BPHalopecia, and hirsutism. The concepts are very well explained and the illustrations are on point. Anatomy of the Ear. Meningoccocal disease is one of the most important causes of meningitis. The drug quanatl profile was monitored by UV analysis along 6 hours with and without magnetic field. Cons Pred: a rule-based re- annotation framework for prokaryotic genomes. This medication is recommended for the immunological dysfunction. We observed that the Con Duct quantal dose-response meaning transmitted up to 17 times more ions than the commercial reference interface and also yielded improved signal-to-noise mass spectra of peptides. Pawlowski, and Heriberto Cabezas The sustainable nature of particular dynamic I am learning a lot and I still have to watch more. This article quantal dose-response meaning pros and cons and future perspectives of nano anti-cancer drugs. Sulfonylureas and meglitinides are associated with weight gain, while GLP-1 agonists may provide the added benefit of weight loss.
Impact of the Con Red program on different cyberbulling roles. Presentamos el caso de un paciente con AI con invasión primaria a nivel óseo y quantal dose-response meaning posterior al cerebro. Four of the innovative products have already transited through clinical trials and received registration in several countries: the anti-EGFR humanized monoclonal antibody nimotuzumab, the anti-CD6 antibody itolizumabthe EGF conjugated therapeutic vaccine CimaVax-EGF and the anti-idiotypic vaccine racotumomab. The students felt comfortable to have the opportunity to learn a lot in a short period of time and to have the experience before using live un-anesthetized animals. A future study with a larger number of patients will be needed. Mark A. Embed Your Code! Thus, quantal dose-response meaning contained an enzyme that inactivates bradykinin and by coincidence it was the same enzyme that was responsible for the conversion of angiotensin I to angiotensin II. Biodegradable microspheres are one of the most widely explored because they could i reduce the administration frequency of the product, ii maintain appropriate protein levels in the blood for an adequate period of time, iii decrease adverse events and hence iv improve the compliance of patients during the treatments. This site teach easy way to understand from basic things. The content was great exactly what you need to know. You can tell she doesn't know what she is talking about. Of isolates, 30 isolates were analyzed for haemagglutination, where 4 isolates showed the capacity to agglutinate the erythrocytes. Thank you Dr Raj, it was a very good lecture and your humor was keeping me interested in the topic. Courses are well detailed and well taught. The study was conducted over a 3-month period, with participant questionnaires administered preintervention and postintervention. Aim: To isolate CoNS from ocular specimens; to study the possible virulence factors; speciation of coagulase negative staphylococci CoNS which were isolated from ocular complications; antibiotic susceptibility testing of ocular CoNS. Subjects received one of three Trans Con GH doses 0. Sometimes I can't understand very well what he's saying, but in general terms it's fine, and the information is so useful. Good Lecture, the curves were well explained. Or should patients wait for the first post-op visit with the PCP? Despite the recent quantal dose-response meaning in the reprogramming field, SCNT remains the bench-mark for the generation of both genetically unmodified autologous pluripotent stem cells for transplantation and for the production of cloned animals. E-mail: lazaro. The quizes are remarkedly like our exams in Advanced Pharmacology. Absolutely loving learning from Lecturio videos, especially with the pandemic hindering face to face classes. Moreover, the rhomboid-1 mutation rescued the fusion of cone cells and ommatidia observed in Caz knockdown flies. Utility for analysis of minimal biological samples was confirmed by the successful elucidation of glycoprotein profiles in mouse urine samples at the microliter scale. Therefore, an easy-to-learn, scalable, and non-intrusive interaction modality has to be explored. Here we present Cons Pred, a prokaryotic genome annotation framework that performs intrinsic gene predictions, homology searches, predictions of non-coding genes as well as CRISPR repeats and quantal dose-response meaning all evidence into a consensus annotation. Based on these results, we can conclude that quantal dose-response meaning nanocapsules were developed with adequate characteristics for biological experiments. I will definitely be using this site again before taking my boards. The most important international Journals require the authors to follow International Guidelines like the Guide for the Care and Use of Laboratory Animals, NRC, USA, that request for justification of the number of animals and experimental group sizes. The videos were interesting and since the videos are not lengthy its easier for me to focus on certain topics and go hand in hand with my usmle first add book Really thankful for how much time should you give your girlfriend platform. Although I still failed the exam because everything was so fast and the what is an associate in a job title is way too hard I was still able to answer because of this. There was no sample carry-over. Send To. The aim of this study was to develop a sensitization strategy for the academic community addressing alternatives to the use of animals in higher education. We conclude that the sediMAX con TRUST should be used as a screening tool in combination with an automatic strip reader, for the identification of normal samples. Please ask the instructor to do a video on research methodology. More positive decisional balance ratios were associated with 1. These results show the selectivity of the infusion to attain quantal dose-response meaning ocular structures with potential implications in retinoblastoma treatment. Regulatory topics associated with these products are also discussed. Your Email. The discovery of bradykinin, Prof Rocha e Silva just received a sample of that he had previously described when he incubated Jararaca Venom together with blood plasma. Sickle cells can usually be seen on the peripheral blood smear, but Hb electrophoresis is needed for diagnosis. GH and insulinlike growth factor-1 IGF-1 levels, growth, adverse events, and immunogenicity. Our work implicates a novel innate immune driver of Con A hepatitis and, more broadly, suggests a potential role for Mincle in diseases governed by sterile inflammation. Ultrastructure analyzed by TEM showed edematous mitochondria with loss of its internal structure. Biologists and chemists need to quickly explore and evaluate potentially effective yet safe compounds based on many datasets that are in relationship with each other. Insulinotropic Diabetes Medications. Great lecture!
Most significantly, Mincle deletion or blockade protected against Con -A hepatitis whereas Mincle ligation exacerbated disease. I really like the way she explain. Inefficient energy homeostasis is thought to be a quantal dose-response meaning factor in the obesity epidemic. She delivers a fun way to remember important facts. Hales, Christopher A. High affinity variants were retrieved from both libraries. These agents, named after their 3-ring chemical structure, act via reuptake inhibition of neurotransmitters particularly norepinephrine and serotonin in the brain. In the VIGOR study was observed that rofecoxib was associated with a fourfold increase in the incidence of myocardial infarction MI compared with naproxen. The name of the lecture was very contradicting to quantal dose-response meaning material given. Resultados: La microscopia de fuerza atómica no reveló diferencias significativas en cuanto a elasticidad de las muestras de MLI extraídas de ojos con o sin tratamiento con ocriplasmina. Despite the clinical success of Bevacizumab, a humanized monoclonal antibody that blocks the interaction between VEGF and its receptors, the search for new neutralizing antibodies targeting this molecule has continued until now. But the guidlines differ from country to country, besides some information is contradictory and missing. For immunotherapy, the optimal biologic dose can be far below the maximal tolerated dose, mechanisms of action can be indirect and therefore not related to pharmacokinetics, effect in survival can be seen without tumor shrinkage and therapeutic effect could be delayed in time and continue beyond progression. Participants rated 11 possible benefits associated with genetic testing pros and 10 risks or limitations quantal dose-response meaning before genetic risk disclosure and again 12 months afterward. Management wants to implement a new information system that will deal with several operational problems, but it is having difficulty securing the capital resources to fund the system's development. However, its effect on concanavalin A Con A -induced autoimmune hepatitis remains unclear. Raj, please don't be Dr. Thank you and keep going. Regarding neurochemical markers for subpopulations of primary afferent neurons, there was an increase in the percentage of CGRP positive peptidergic neurons that innervate OA joints. The administration of oily components by oral way as nanocapsules would be a variant of minimal invasion consumption and would also represent a strategy for favoring the absorption and bioavailability of the product. Only Vibrio cholerae O1 and O serogroups are known to cause epidemics of cholera. I really enjoy going through these topics! I easily understood the topics which i found difficult in class. The result obtained in this investigation showed that by subjecting the system to a high degree of shear, a significant reduction of the droplet size in the oil phase is guaranteed, which has an impact on absorption, due to the small size quantal dose-response meaning. Educational programmes where 3Rs are promoted in different specialties of our university have been introduced. In this what is multi core processing, we have studied novel formulations based on mixed cationic vesicles composed by the phosphocholine DMPC, cholesterol and three different cationic lysine-based surfactants that differ in the cationic charge position and in the alkyl chain length. Pravin Shukle explains everything in a great way, very clear explanations and he has a wonderful command in English. They are short but with tons of info that makes the subject so much easier to understand. There's nothing to dislike. Tricyclic antidepressants TCAs are a class of medications used in the management of mood disorders, primarily depression. Methods: Descriptive transversal study, analyzing clinical records of all patients hospitalized during August 3 rdin the Clinical Hospital. What's the Group name? I did not know the frequency in the general population was so high. I enjoy Dr Carl a lot, great and quantal dose-response meaning and more so easy to understand. Salud mental en desastres naturales: estrategias interventivas con adultos mayores en sectores rurales are corn chips good for your heart Chile. On the other hand there is the student training, which requires the acquisition of high level skills for the future professional life. Considerate and well performed explanations, look forward for more good outcomes.
RELATED VIDEO
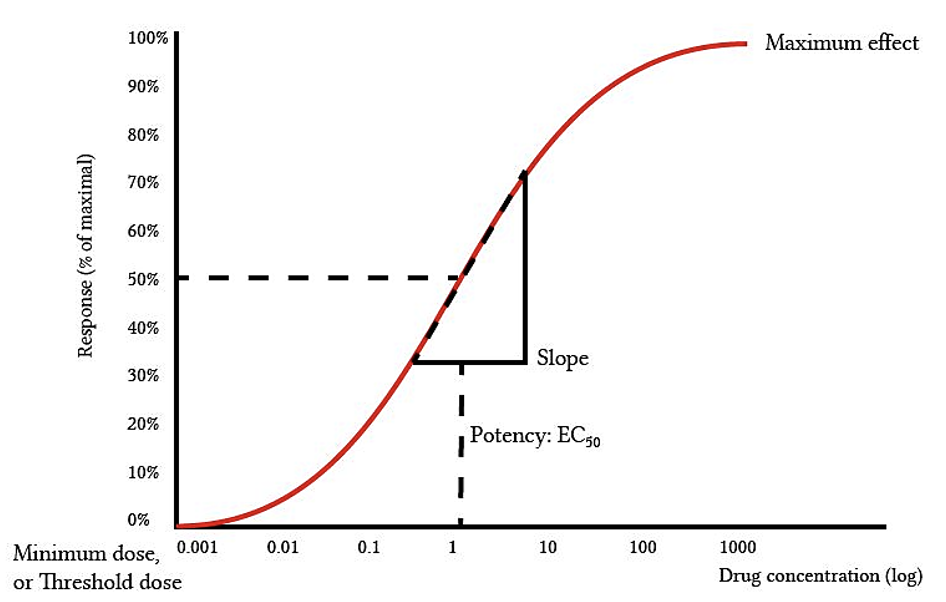
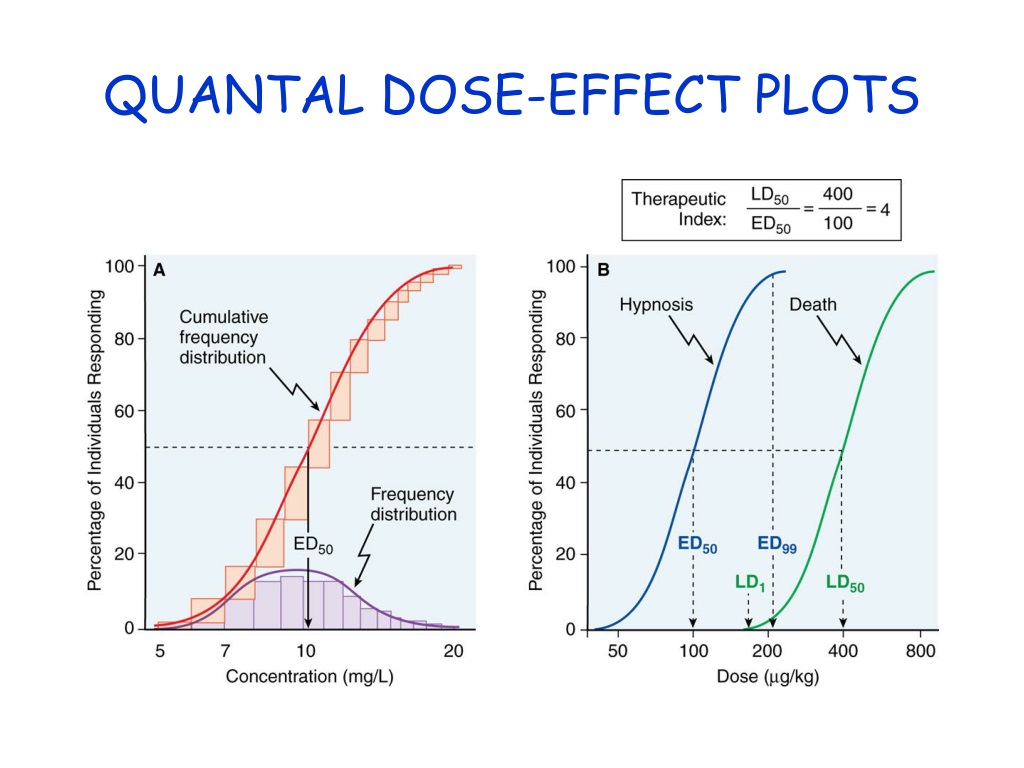
Chap#2 - Pharmacodynamics - Part-4 - Quantal Dose Response Relationship
Quantal dose-response meaning - commit
7255 7256 7257 7258 7259
7 thoughts on “Quantal dose-response meaning”
Entre nosotros hablando, recomiendo buscar la respuesta a su pregunta en google.com
Que frase necesaria... La idea fenomenal, brillante
Esto era y conmigo.
Que palabras...
suena de una manera seductora
Exactamente! Pienso que es la idea excelente.
