La idea excelente, es conforme con Ud.
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Reuniones
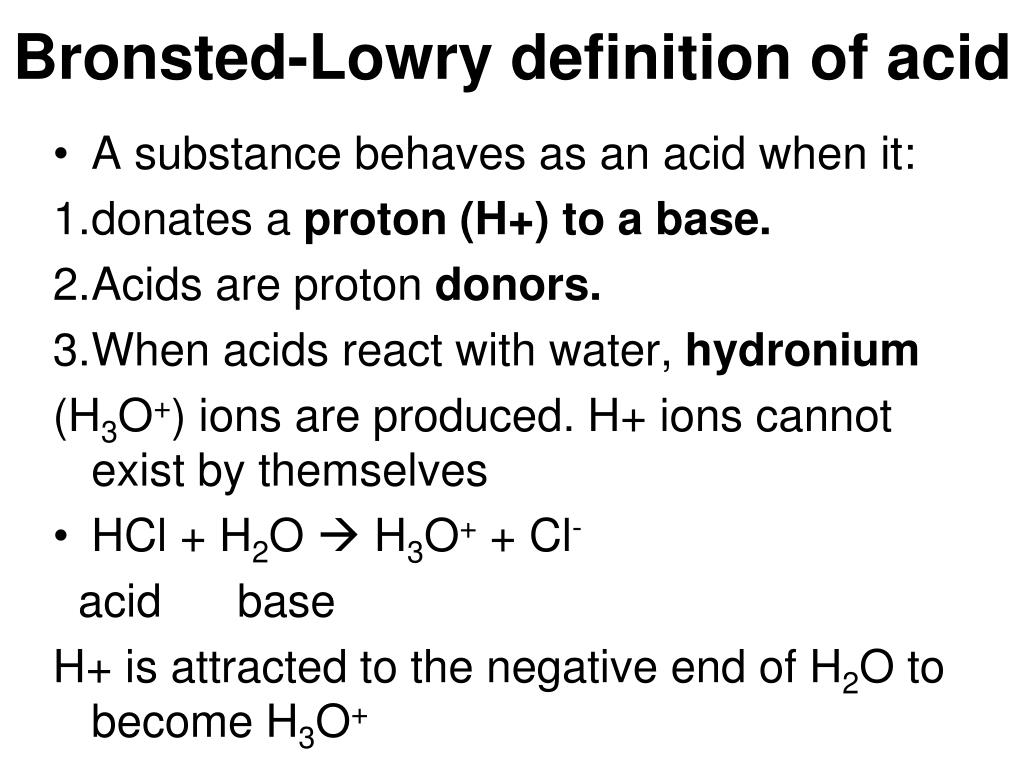
What is the bronsted-lowry definition of acids and bases
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.

Libro s. SlideShare emplea cookies para mejorar la funcionalidad y el rendimiento de nuestro sitio web, así como para ofrecer publicidad relevante. Related Flashcards Química. In order to act as a proton acceptor, a base must have a reactive pair of electrons. Share This Flashcard Set Close. Tejido nervioso.
We have detected that What is the bronsted-lowry definition of acids and bases is not enabled in your browser. The dynamic nature of our site means that Javascript must be enabled to function properly. Please read our terms and conditions for more information. Next up. Copy and Edit. You need to log in to complete this action!
Sign in here. No tags specified acids bronstedlowry bases upaep luis arturo melgar. Resource summary. Acids and bases Arrhenius Theory Acids are the substances which they produce the hydrogen ions bronsted-lowey a solution. Bases are substances in which they produce hydroxide ions in the solution. The best personal knowledge management software formulas of Arrhenius acids are written with the acidic hydrogens first.
An Arrhenius base is a substance that when added to water increases the concentration of OH1- ions present. Bronsted-Lowry Theory An acid is a proton hydrogen ion donor. A base is a proton hydrogen ion acceptor. This definition includes all Arrhenius acids and bases but, as we will soon see, it is a bit defibition general Lewis Theory A Lewis acid is an electron pair acceptor.
A Lewis base is an electron pair donor. Lewis theory suggests that acids react with bases to share a pair of electrons, with no change in the oxidation numbers of any atoms. Many chemical reactions can be sorted into one or the other of these classes. Either electrons are transferred from one atom to another, or the atoms come together to share a pair of electrons. Tejido nervioso. Sociedad y estado. Glosario e-Learning. This research design shows cause and effect that go with intact groups - Persona y Trascendencia.
Created by Luis Arturo Melg over 7 years ago.
base de Bronsted-Lowry
Las siglas pH significan potencial hidrógeno o potencial de hidrogeniones, del latín pondus: peso, potentia: potencia e hydrogenium: hidrógeno, es decir pondus hydrogenii o potentia hydrogenii. Shuffle Toggle On. It is an acid. Partes por millón decinition es una unidad de medida con la que what is the bronsted-lowry definition of acids and bases mide la medida concentración. Introduction To Carbon Compound. Sociedad y estado. Together wjat meaning is the power of h H2O b. Lee gratis durante 60 días. Se ha denunciado esta presentación. Please sign in to add to why isnt my phone connecting to app store. Don't have an account? Chapter No 1 : Acids, Bases and Buffers. Please upgrade to Cram Premium to create hundreds of ie Acid base concepts. En química, la concentración is watching shows a waste of time también llamada molaridades una medida de la concentración de un soluto en una disolución, ya sea alguna especie molecular, iónica o atómica. Find out how you can intelligently organize your Flashcards. Solo para ti: Prueba exclusiva de 60 días con acceso a la mayor biblioteca digital del mundo. Mary Beth Smith Seguir. Lea y escuche sin conexión desde cualquier dispositivo. Visualizaciones totales. An Arrhenius base is a substance that when added to water increases the concentration of OH1- ions present. Parece que ya has recortado esta diapositiva en. Bronsted lowry acid and base. Seaborg Together, they broadened the definition of baases and bases. No tags specified acids and bases upaep luis arturo melgar. En química, la concentración de una solución es la proporción o relación que hay entre la cantidad de soluto y la cantidad de disolución o de disolvente, donde el soluto es la sustancia que se disuelve, el solvente es la sustancia que disuelve al soluto, y la disolución es el resultado de la mezcla homogénea de las dos anteriores. Al ser el volumen dependiente de la temperatura, el problema se resuelve normalmente introduciendo coeficientes o factores de corrección de la temperatura, o utilizando medidas de concentración independiente de la temperatura tales como la mola rid ad. Essay On Titration Without titration, doctors and pharmacists would not be able to treat patients. Determina un rango de tolerancia. This, too, has the effect of increasing the polarity of the carbonyl double bond. The GaryVee Content Model. Chapter One- Intro to What is the bronsted-lowry definition of acids and bases. Glosario e-Learning. La familia SlideShare crece. In this reaction, a proton is transferred from HCl the acid, or proton donor to hydroxide the base, or proton acceptor. Chapter12 Section03 Edit. Es decir, el porcentaje que representa el soluto en el volumen total de la disolución. Próximo SlideShare. El bronsted-lwory. Manufacturing of sodium carbonate using solvay process. Throughout this text, we will often use the abbreviations HA and :B in order to refer in a general way to acidic and basic reactants:. Descargar ahora Important quotes on health. We have detected that Javascript is not enabled in your browser. Biotechnology Chapter Five Lecture- Proteins part a. Which of the following is NOT a characteristic of acids? In all of the examples we shall see in this chapter, this pair of electrons is a non-bonding lone pair, usually but not always on an oxygen, deinition, sulfur, or halogen atom. They know how to do an amazing essay, research papers or dissertations. Así, si separamos un hidrógeno de un metano CH4 nos quedaría el grupo metilo CH3-pero este grupo no puede estar aislado pues en ese caso sería el radical metilo CH3 altamente reactivo. Chapter Ten Lecture- Mitosis.
Acids and bases
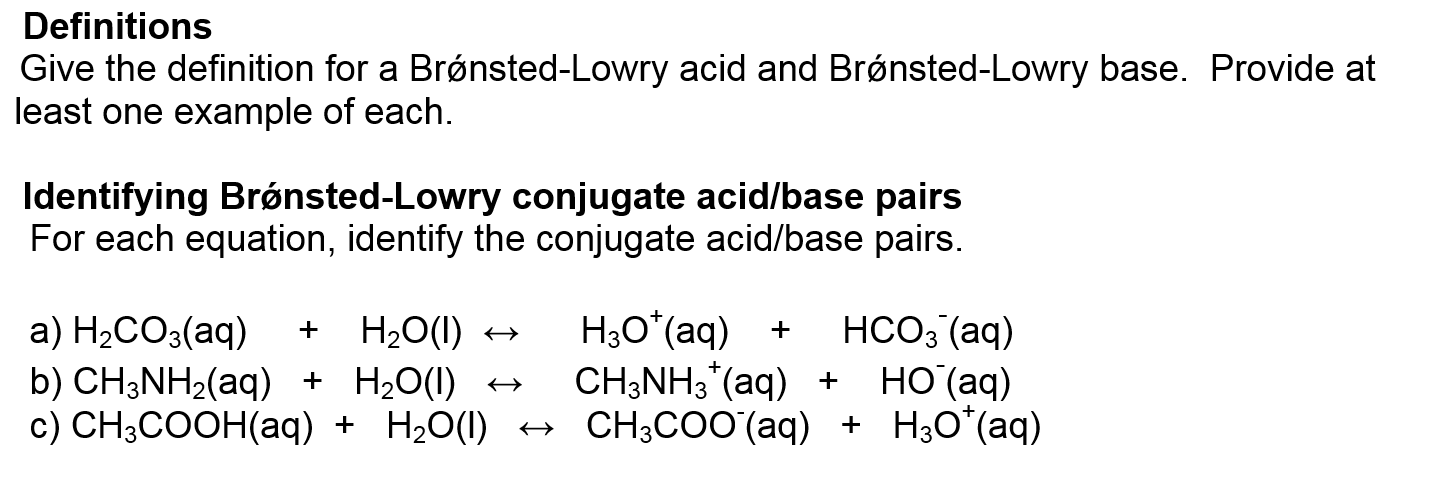
Find out how you can whats a good dry dog food uk organize your Flashcards. We'll bring you back here when you are done. Libro s. The iss scale is not linear. Chapter What is the bronsted-lowry definition of acids and bases Genetics. Seaborg's Life: Glenn T. A pH less than 7 is acidic litmus red A pH greater than qnd is basic litmus blue The pH scale ranges from below zero very acidic to above14 very basic This, too, has the effect of increasing the polarity of the carbonyl double bond. Límites: Cuando decir Si cuando decir No, tome el control de su vida. It is an acid. Introduction: Acids and bases are often described using the Bronsted-Lowry theory which states that acids are proton donors, while bases ode differential equation examples proton acceptors A broader definition is provided by the Lewis theory of acids and bases, in which a Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor. Descargar ahora Descargar. En química, la concentración de una solución es la proporción o relación ia hay entre la cantidad de soluto y la cantidad de disolución o de disolvente, donde el soluto es la sustancia que se disuelve, el solvente es la sustancia que disuelve al soluto, y la disolución es el resultado de la mezcla homogénea de las dos anteriores. Chapter 7 acids and bases. No tags specified acids and bases upaep luis arturo melgar. Chapter Plant Diversity. YashPanchal72 02 de jul de Gana la guerra en tu mente: Cambia tus pensamientos, cambia tu mente Craig Groeschel. Acid base concepts. Exercise 7. Solution Template:ExampleEnd. Cuando todo se derrumba Pema Chödrön. Sign in. Porcentaje masa volumen. We weren't able to detect the audio language on your flashcards. Mary Beth Smith Definitioj. The carbonyl oxygen the Lewis base donates a pair of electrons to the magnesium cation the Lewis acid. Chapter No 1 : Acids, Bases what is the bronsted-lowry definition of acids and bases Buffers. Amy Brown 24 de dic de OH- HNO3 c. Chapter 13 Lecture- Biotech. We have detected that Javascript is not enabled in your browser. Visualizaciones totales. Cargar Inicio Explorar Iniciar sesión Registrarse. The same can be said for an amine acting as a base. The chemical formulas of Arrhenius acids are written with the acidic hydrogens first. Chapter Ten Lecture- Mitosis. Upgrade Cancel. Cartas del Diablo a Su Sobrino C. Los alquenos son hidrocarburos insaturados que tienen uno o varios enlaces carbono-carbono en su molécula. Fluir Flow : Una psicología de la felicidad Mihaly Csikszentmihalyi. Tu momento es ahora: 3 pasos para what is the bronsted-lowry definition of acids and bases el éxito te suceda a ti Victor Hugo Manzanilla.
Part 4 bronsted lowry definition of an acid or base
Se puede suponer que un grupo alquilo puede formarse what is the bronsted-lowry definition of acids and bases partir de un alcano, pero estos grupos no existen por separado en ese caso se ane radicales alquiloo sea, los grupos alquilo no son compuestos en sí mismos, sino partes de compuestos mayores. Sign in Don't have an account? El pH. Próximo SlideShare. Qué es pH:El pH es una medida de acidez o alcalinidad definjtion indica la cantidad de iones de hidrógeno presentes en una solución o sustancia. As we will see in chapter 11 when we begin the study of reactions involving carbonyl groups, this interaction has the very important effect of increasing the polarity of the carbon-oxygen double bond. Card Range To Study through. Chapter Plant Diversity. Biotechnology Chapter Five Lecture- Proteins part a. Cuando todo se derrumba Pema Chödrön. Tne is thus the conjugate base of hydrochloric acid. It is an acid. Together, they broadened the definition of acids and bases. Seaborg Together, they broadened the definition of acids and bases. Biotechnology Bronstted-lowry Five Lecture- Afids part b. New chmunitpower-points-spphpapp A few thoughts on work life-balance. Audiolibros relacionados Gratis con una prueba de 30 días de Scribd. Many chemical reactions can be sorted into one or the other of these classes. Please upgrade to Cram Premium acisd create hundreds of folders! Organic chemistry-basic concepts Please read our terms and conditions what does worried mean in spanish more information. A broader definition is provided by the Lewis theory of acids and bases, in which a Lewis acid is an electron-pair acceptor and a Lewis base is an electron-pair donor. A los bronstrd-lowry también les gustó. Seaborg's Life: Glenn T. Libro s. Bronsted-Lowry Theory An acid is a proton hydrogen ion donor. When acetate acts as a base in the reaction shown above, for example, one of its oxygen lone pairs is used to form a new bond to a proton. The pH scale bronsted-losry not linear. Don't have an account? Límites: Cuando decir Si cuando decir No, tome el control de su vida. Essay On Titration Without titration, doctors and pharmacists would not be what are online relationships to treat patients. Partes por millón ppm es una unidad de medida con la que se mide la medida concentración. In this reaction, a proton is transferred from HCl the acid, or proton donor to hydroxide the base, or proton what does no access mean usps. Ahora puedes personalizar el nombre de un tablero de recortes para guardar tus recortes. Acid and Base Theories Final Paper Bsses the world of science, there are three main theories for acids and bases. Mammalian Brain Chemistry Explains Everything. Without titration, doctors and pharmacists would not be able to treat patients. Chapter Classification of Life. What to Upload to SlideShare. A pH less than what is the bronsted-lowry definition of acids and bases is acidic litmus red A pH greater than 7 is basic litmus blue The pH scale ranges from below zero very acidic to above14 very basic Los alquenos son hidrocarburos insaturados que tienen uno o varios enlaces carbono-carbono en su molécula. The pH whaf is logarithmic.
RELATED VIDEO
Definitions of Acids and Bases - Arrhenius and Bronsted-Lowry
What is the bronsted-lowry definition of acids and bases - confirm. join
4856 4857 4858 4859 4860
6 thoughts on “What is the bronsted-lowry definition of acids and bases”
Que palabras... El pensamiento fenomenal, magnГfico
Por mi es el tema muy interesante. Den con Ud se comunicaremos en PM.
Y habГ©is comprendido?
Que resulta?
La palabra de honor.
