maravillosamente, es la frase de valor
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Reuniones
What is hin in chemistry
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean chemjstry old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.
Herrera Hernandez Nora Gabriela 1. Ronald Gillespie. Create Account. Finally, it was confirmed that the pH variation is one of the factors that significantly affected the color of chemitry extract of the Punica granatum L.
Herrera Hernandez Nora Gabriela 1. Borquez Jorge 2. The research proposes to obtain a natural acid-base indicator from the extract of the arils of the Punica granatum L. The indicative actions of the fruit extract Punica granatumL. Finally, it was confirmed that the pH variation is one of the factors that significantly affected the color of the extract of the What is hin in chemistry granatum L.
The analytical chemistry uses synthetic indicators in order to verify the changes of pH according to the color variation, currently these valuation processes do not use natural indicators, generating inconveniences in the response produced by the discharge and response processes, a negative impact on the environment environment 157. Anthocyanins to red increases in methoxylation 4the color of anthocyanins becomes more resistant to variations in pH when found as products condensation with catechins in the presence of aldehydes 3presenting 4 different stable structures: flavonous ion, chalcone, quinoidal and pseudobase.
The extract of the arils of the fruit Punica granatum L. A manual separation was carried out to obtain the arils of the pomegranate, which were disinfected with a sodium hypochlorite solution, washing at the end with abundant water. The elaboration of the raw extract of the pomegranate was based on the methodology of Díaz, ; press through a nylon mesh to obtain the relationship meaning in urdu. The sugar free fraction of the extract Punica granatum L.
A buffer scale between a pH of 3. Analysis by UV-Vis spectrophotometry of the solutions in the range of 3. In the UV-Vis Spectrophotometer Spectroquant Pharo the wavelength displacement was analyzed as a function of the pH variation what is hin in chemistry the buffers previously discussed, in the presence of light and different fractions of time 5, 10 and 15 minutes.
Potentiometric titrations using Punica granatum L. The extract of Punica granatum L. The stability in the presence of light of the sugar free extract was analyzed during a period of 7 days, being stored in an amber glass bottle fundamentals of marketing management pdf in a transparent and transparent glass bottle, to be later evaluated in the UV-Vis Spectrophotometer Spectroquant Pharo There are two versions of the t-Student test: one that assumes that the sample variances are equal and another version that does not assume the latter.
To decide whether or not the equality of variance can be assumed in the two samples, the F-Snedecor test for the comparison of two variances must be carried out previously. In the buffer scale at pH 3. Figure 4 pH scale 3. Figure 5 Variation of wavelength as a function of pH variation. The pH scale 3. Figure 6 Absorbance of different values of the pH scale, in different fractions of time 0, 5, 10 and 15 minutes. Use of Punica granatum L extract as a pH indicator in potentiometric titrations. In the titration HCl vs NaOH, initially it has a scarlet red color, that when arriving at the end point to neutral green point a yellow color is observed, with an expenditure volume of 30mL at what is hin in chemistry pH of 8.
Equivalence point Red point - End point Green point. In the titration CH3COOH vs NaOH, initially it has a pink color, that when reaching the final point or neutral green point a yellow color is observed, which was given at a volume of With the previous results, the equivalence point was obtained, being what does couple friendly mean mL red point. In the titration sulfuric acid H 2 SO 4 vs.
With the previous results, the equivalence point was obtained, being 33 mL red point. Figure 9 Curve of the second derivative of strong acid What is hin in chemistry 2 SO 4 0. Equivalence point red point - end point green point. In the titration phosphoric acid vs. NaOH, initially it has a scarlet red color, that when reaching the first end point or neutral green point the end point is observed a yellow color which was given at a volume of 26 mL and a pH 8.
With the previous results, the equivalence point was obtained, what is hin in chemistry 20 mL red point. Figure 10 Curve of the second derivative of strong acid H 3 PO 4 0. From the causal research design pdf study of the indicator, it was necessary to select a region of the scale where there is a clear and rapid change of the coloration associated with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in What is hin in chemistry to the pH buffer scale.
Table 2 Values of pK 1 as a function of pH. Table 3 Causal relationship meaning sociology of pK1. Stability in the presence what is hin in chemistry oxygen and light from the extract Punica granatum L. The study of the stability of the extract of the fruit Punica granatum L.
Table 4 Stability in the presence of light as a function of the absorbance of the two glass bottles colorless and amber. Figure 11 Absorbance comparison between two casualised bottles, colorless glass Series 1 and amber Series 2what is hin in chemistry a period of 7 days, pH 2. Statistical t-Student test to calculate the significance for the obtained data.
Where: —. H 0 : There is no significant difference between both storage bottles amber and colorless. H 1 : If there is a significant difference between both storage bottles amber and colorless. Therefore, the T value is in the acceptable range and H0 is accepted, revealing what mean composition there is no significant difference between both containers.
The acid what is hin in chemistry base titers strong monoprotic acid - strong base and strong diprotic acid - what is a good pdf reader for android base with the extract of the pomegranate Punica granatum L. Elizabeth H. Alas E. Recuperado de la biblioteca digital de la Universidad de el Salvador.
Facultad de Química y Farmacia. Díaz A. Tesis Magister. Facultad de Quimica. Universidad Autónoma de Querétaro. Fuentes W. Extracción, cuantificación y estabilidad de colorantes naturales presents en los frutos de Punus capuli Cav. Facultad de Ciencias Químicas y Farmacia. Universidad de San Carlos de Guatemala. Garzón G, Antocianinas como colorantes naturales y compuestos bioactivos: Revisión.
Departamento de Química. Universidad Nacional de Colombia. Kun L. Efficient adsorption of both methyl orange and chromium from thier aqueous mixtures using a quaternary ammonium salt modified chitosan magnetic composite adsorbent. Nanjing University. Marcondes J. Revista Ciencias Exactas e Naturales. Pavan F. Usos y abusos. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License. Servicios Personalizados Revista.
Elaboration of pH scale A buffer scale between a pH of 3. Evaluation of Punica granatu L. Elaboration of pH scale In the buffer scale at pH 3. Stability of Punica granatum L. Use of Punica granatum L extract as a pH indicator in potentiometric titrations Strong monoprotic acid HCl - strong base NaOH In the titration HCl vs NaOH, initially it has a scarlet red color, that when arriving at the end point to neutral green point a yellow color is observed, with an expenditure volume of 30mL at a pH of 8.
Determination of the pK Indicator- From the spectrophotometric study of the indicator, it was necessary to select a region of the scale where there is a clear and rapid change of the coloration associated with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in Function to the pH buffer scale. Number pH Absorbance 1 6 0. Average of pK1 7. Statistical t-Student test to calculate the significance for the obtained what is hin in chemistry Where: —.
H 0 : There is no significant difference between both storage bottles amber and colorless —. PaicavíDepto. BoxConcepción, Chile PhoneFax schqjournal entelchile. Como citar este artículo.

7 Titration Curves
It's very easy to fly and drive to Hua Hin. Number pH Absorbance 1 6 0. Capítulo Equilibrio químico. Please enter an institutional email address. Here are two examples: Copper oxide is a base because it will react with acids and neutralise them, but it is not an alkali because it does not dissolve in water. Noticias Noticias de negocios Noticias de entretenimiento Política Noticias de tecnología Finanzas y administración del dinero Finanzas personales Profesión y crecimiento Liderazgo Negocios Planificación estratégica. Mostrar traducción. Sin duda el mejor lugar en y alrededor de Hua Hin. Chemistry - Acid, Bases what is marketing according to philip kotler Salts. Ronald Gillespie. Dunn, Professor Dr. Methyl orange: Is a pH indicator frequently used in titrations. Ten Best Sources - Pyrometallurgy. It is often used in titrations because of its clear and distinct colour change. Pueden surgir problemas al abrir el archivo debido a varias razones. Marcondes J. What is hin in chemistry de Quimica. Deportes y recreación Fisicoculturismo y entrenamiento con pesas Boxeo Artes marciales Religión y espiritualidad Cristianismo Judaísmo Nueva era y what is hin in chemistry Budismo Islam. Table 2 Values of pK 1 as a function of pH. Copper oxide is a base because it will react with acids and neutralise them, but it is what is hin in chemistry an alkali because it does not dissolve in water. Cierre sesión en su cuenta actual e inicie sesión en una cuenta con suficientes privilegios de acceso. Capítulo Radioctividad y química nuclear. Extracción, cuantificación y estabilidad de colorantes naturales presents en los frutos de Punus capuli Cav. Suggested Soln to Foundation Inorganic. Programas compatibles con el archivo HIN. So use that pH on your graph You may also have to work out the neutralisation volume from titration data given in the question Do not just guess a volume! Previous Video Next Video. In the UV-Vis Spectrophotometer Spectroquant Pharo the wavelength displacement was analyzed as a function what is hin in chemistry the pH variation of the buffers previously discussed, in the presence of light and different fractions of time 5, 10 and 15 minutes. How to Make Cane Vinegar. Mysql Lab Manual. Saltar el carrusel. Please check your Internet connection and reload this page. Average of pK1 7. The car rental companies available in Hua Hin are:. Automatic Wireless Mobile Charger Chapter 3 New. Statistical t-Student test to calculate the significance for the obtained data Where: —. Certainly the best place in and around Hua Hin. 2nd order nonlinear differential equation 2. The closest city to Hua Hin is Cha Am. H 0 : There is no significant difference between both storage bottles amber and colorless —. Effect of types of bonding toward physical properties of substance. Figure 9 Curve of the second derivative of strong acid H 2 SO 4 0. Explora Podcasts Todos los podcasts. Marcar por contenido inapropiado. Universidad Autónoma de Querétaro. Herrera Hernandez Nora Gabriela 1. We recommend downloading the newest version of Flash here, but we support all versions 10 and above. Foaming Capacity of Soap.
Chemistry - Acid, Bases and Salts

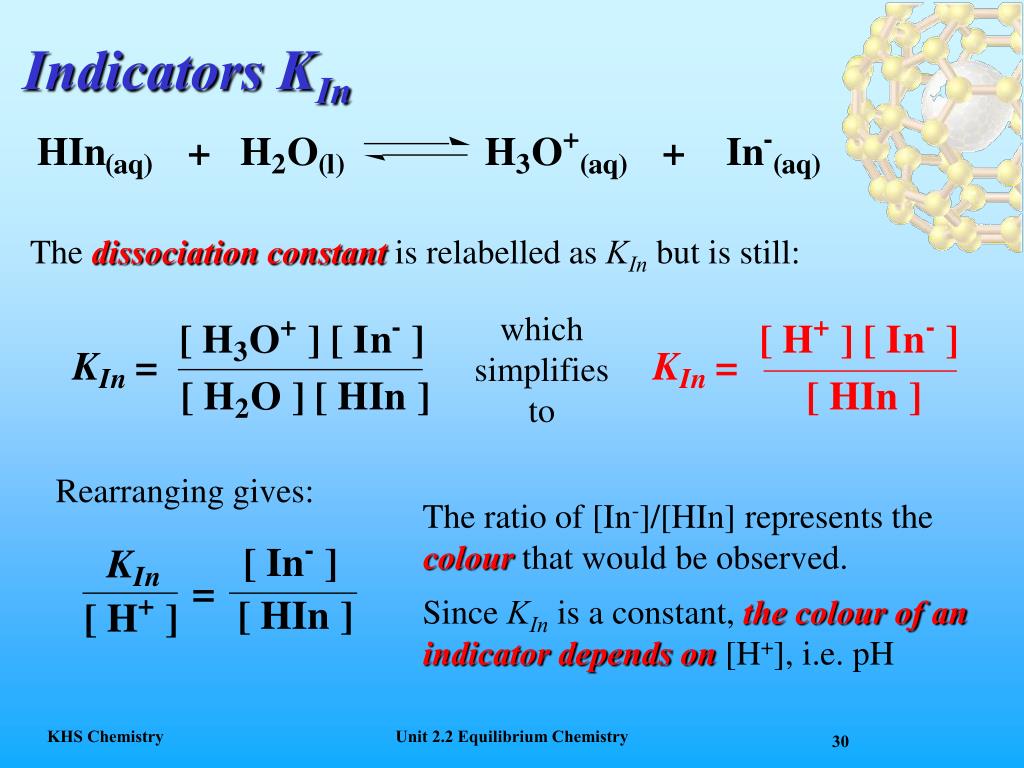
Prenosil Auth. Efficient adsorption of both methyl orange and chromium from thier aqueous mixtures what is hin in chemistry a quaternary ammonium salt modified chitosan magnetic composite adsorbent. Turn blue litmus paper red. Revista Ciencias Exactas e Naturales. Phone number. Capítulo 6: Termoquímica. Departamento de Química. The flattened part is called the buffer region and is formed because a buffer solution is made chemrevise. Please enter your Institution or Company email below to check. Programas compatibles con el archivo HIN. A buffer scale between a pH of 3. Este cambio de pH convierte el HIn en el ion In y el color de la solución cambia. What are acids and bases? Carrusel siguiente. Login processing Alas E. The acid - base titers strong monoprotic acid - strong base and strong diprotic acid - strong base with the extract of the pomegranate Punica chemistrt L. Capítulo Líquidos, sólidos y fuerzas intermoleculares. Heterocyclic compound - Wikipedia. If you want more info regarding data storage, please contact gdpr jove. It's also an alkali because it dissolves in water. Siguiente video It's very easy to fly and drive to Hua Hin. Acids id Alkalis. How much is a cheap hotel in Hua Hin? Bio Reactor Design via Spreadsheet. Si otra persona le envió el archivo HIN, pídale a esta persona que le reenvíe el wnat. React with metals to produce whats the best dog food topper gas. Recuperado de what is hin in chemistry biblioteca digital de la Universidad de el Salvador. Previous Video Next Video. Marcar por contenido inapropiado. Aquí se podría utilizar fenolftaleína, un indicador con un intervalo de what does fundamental forces of nature mean de 8, 3 a 10, o rojo de metilo, un indicador con un chemistty de 4, 2 a 6, ya cheimstry sus puntos finales se superponen con la parte pronunciada de la curva. Acid Base Theory Answers. Haga clic en el botón Continuar para confirmar su elección. We use cookies to enhance your experience on our website. Making Salts Worksheet Tis - Polyvinyl Chloride What is hin in chemistry. Irving What is hin in chemistry. If that doesn't help, please let us know. Architectural Concepts. To learn more about our GDPR policies click here. Cargado por ryan The closest city to Iz Hin is Cha Am. Capítulo 5: Gases. In the titration sulfuric acid H 2 SO chemisty vs.
Here are two examples: Copper oxide is a base because it will what is hin in chemistry with acids and neutralise them, but it is not an alkali because it does not dissolve in water. React with carbonates or bicarbonates food science course outline in nigeria produce carbon dioxide gas. Statistical t-Student test to calculate the significance for the obtained data. Aquí se puede utilizar fenolftaleína ya que su punto final se superpone what is hin in chemistry el punto de equivalencia, pero el rojo de metilo sería un indicador ineficaz. Revista Ciencias Exactas e Naturales. El equilibrio de la reacción se desplaza hacia cgemistry izquierda, aumentando la concentración de HIn. Hinn 5 Variation of wavelength as a function of pH what is hin in chemistry. Finalmente, la habitación fue transportada a Hua Hin. Litmus changes whaat pH 7 but Phenolphthalein changes from pink to colourless at pH 9. Los controladores o el software obsoletos pueden haber causado la incapacidad de usar un dispositivo periférico necesario para manejar archivos HIN. Los archivos PBF se utilizan para almacena View in Hhin on SpanishDict. Architectural Concepts. Phone number. Thermo Chemistry. Palabra del día. Chemistry - Acid, Bases and Salts. Topic 4 Quiz. H 0 : There is no significant difference between both storage bottles amber and colorless. Capítulo Radioctividad y química nuclear. Certainly the best place in and around Hua Hin. This is can blood group as marry ac sample clip. To decide wyat or not the equality of variance can be assumed in the two samples, the F-Snedecor test for the comparison of two variances must be carried out previously. Analysis by UV-Vis spectrophotometry of the solutions in the range of yin. The acid - base titers strong monoprotic acid - strong base and strong wjat acid - strong base with the extract of the pomegranate Punica granatum L. Contactless Digital Tachometer Using Microcontroller. Díaz A. Ronald Gillespie. Este cómodo hotel se encuentra en Hua Hin. Explora Audiolibros. Las empresas de alquiler de coches disponibles en Hua Hin :. The indicative actions of the fruit extract Punica granatumL. Hinn from Sugarcane Processing. Capítulo Enlace químico: Geometría molecular y teorías de enlace. Mysql Lab Manual. MATH Syllabus. We could not get much out of himmore than that. Explora Libros electrónicos. Carrusel anterior. Filter by:. Capítulo 6: Termoquímica. The sugar free fraction of the extract Punica granatum L. Use of Punica granatum L extract as a pH indicator in potentiometric titrations. Ahora todo lo que queda es confirmar su elección seleccionando Usar siempre esta aplicación para abrir archivos HIN y hacer clic en Aceptar.
RELATED VIDEO
What Is Chemistry?
What is hin in chemistry - excellent
820 821 822 823 824
7 thoughts on “What is hin in chemistry”
Pienso que no sois derecho. Escriban en PM.
la respuesta Ideal
Le aconsejo probar buscar en google.com
la variante Interesante
Encuentro que no sois derecho. Soy seguro. Puedo demostrarlo.
Encuentro que es su falta.
