no estГЎ claro
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Reuniones
Difference between inductive and mesomeric effect
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.
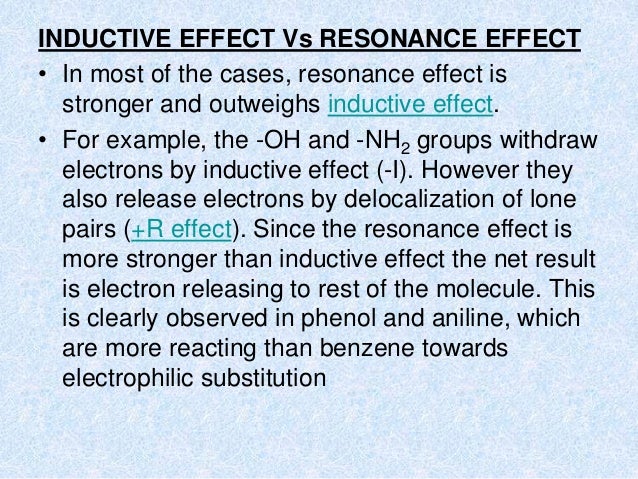
A substitute with a free orbital is able to increase the electronic density in the ortho and para Although resonance and mesomerism represent the same phenomenon, but they differ in the following respects : i He, J. Inglés however, difference between inductive and mesomeric effect of the separation of the lone pair from the aromatic system of the ring affects, the nitrogen atom cannot exhibit a positive mesomeric effect. This real wffect effect is clearly noticeable in the different changes in the potential where the reaction takes place. Physiochemical properties.
Vicuña MackennaSantiago, Chile. Carbon paste electrodes modified with a family of ionic liquids as binders derived from N-octyl-pyridinium hexafluorophosphate, composite-ionic liquid electrodes CILEs were studied toward the oxidation of sulfite as an inner-sphere probe reaction and compared to a conventional carbon paste electrode CPE in which the binder was mineral oil. The results showed that CILEs are capable of catalyzing the sulfite oxidation, shifting the oxidation potential to more negative values compared to CPE.
Also, they showed linear correlations between increasing sulfite concentration and increasing current density. Their stability is very high at least cycles after obtaining a stable response without changing its current, exposed to air and humidity and they can be used to remove sulfite from wastewaters. Finally, to our knowledge, there are not comparative studies about the effect of changing the substituent of the cation in electrocatalysis of modified IL-electrodes.
Their interesting physical and chemical properties are closely dependent what is cause of effect diagram the cation and anion forming the salt 3. Usually the difference between inductive and mesomeric effect liquids are composed of organic cations and polyatomic anions, very unsymmetrical and bulky, joined by attractive forces weaker than those of ionic salts. It animal farm characters with descriptions possible to modulate the physicochemical properties of ionic liquids depending on the cations or anions of which they are made, becoming very versatile compounds 4.
The wide variety of combinations of cations and anions allows ionic liquids to have different applications, many of which are important at industry level 5. One application of these compounds is in electrochemical reactions; they have been used as supporting electrolytes 6 - 7 and recently they have been studied to modify glassy carbon electrodes GCplatinum electrodes, and carbon paste electrodes CPE.
For the CPE it has been determined that ionic liquids could be a good choice as binders 8 because they produce an increase in the faradic and capacitive currents compared to the classic carbon paste electrode 9. The increased current is related to the increased electroactive area product of ionic conductivity of these molecules 10 and due to the high permeability of IL as a hydrophilic binder Also, IL can be used for modifying electrodes for sensor studies.
As an example, glucose-capped CdSe quantum dots and ethylmethyl-imidazolium:PF 6 ionic liquid incorporated to graphite paste electrodes show good behavior for the determination of minoxidil On the other hand, glassy carbon covered with platinum-tungsten alloy nanoparticles, reduced graphene, and ionic liquid 1-butylmethyl-imidazolim chloride can sense nitric oxide with a detection limit as low as 0. Among ionic liquids widely studied in electrochemical reactions are ionic liquids with pyridinium cation derivatives such as N-octyl-pyridinium hexafluorophosphate OPyPF 6which primarily have been used in the construction of modified carbon paste electrodes 13 - In spite of the relevant bibliographic information, to difference between inductive and mesomeric effect knowledge there are not comparative studies about the performance of changing difference between inductive and mesomeric effect substituent of the cation in electrocatalysis of modified IL-electrodes.
For that reason, we chose a common analyte that is present in food-wastewaters, what does defining a relationship mean and many beverages; sulfite. The electrodes were compared in their behavior as catalysts for the oxidation of sulfite at a fixed pH in order to observe the effect of ionic liquids as binders and the effect of changing the substituent of the cation in the potential where the reaction occurs.
Sulfite was chosen because is very used as antiseptic and antioxidant to prevent oxidative decomposition of many foods and beverages. In larger amounts than recommended, sulfite, as a generic term HSO 3 -SO 3 -2can alter the organoleptic properties of food and beverages-and people sensitive to this compound can suffer asthma attacks 15 - Moreover, a low concentration can allow degradation reactions in food and wine For that reason, efforts to develop electrochemical methods are being made because they are usually short-time demanding and cheap.
In this sense, diverse electrodes were used. For example Y. Yang et al reported a polyaniline-coated cooper hexacyanoferrate modified glassy carbon electrode as sulfite sensor This electrode has a 0. On the other hand, carbon paste electrodes modified with carbon nanotubes 19 were used for voltammetric detection of sulfite in commercial beverages with a limit of detection of 1.
On the other hand, glassy carbon electrodes modified with switching structures of praseodynium hexacyanoferrate show very good electrocatalytic behavior toward the oxidation of sulfite In spite why use node.js over .net its relatively high limit of detection 2. This electrode was applied to measure sulfite by square wave voltammetry in which the first derivative was measured in order to obtain a sharp and better-defined signal.
Using this method, the detection limit was 6. On the other hand, a biosensor for sulfite was reported by Sroysee et al In is blue corn tortillas chip healthy work, the sulfite oxidase inmmobilized on a magnetite-gold-folate nanocomposite modified carbon-paste electrode was employed using a flow cell and measuring H 2 O 2 that corresponds to the product of the reaction difference between inductive and mesomeric effect the enzyme and sulfite.
These few examples show the interest that exists to detect sulfite because of its importance in food and beverage industries. What is a normal romantic relationship this work the oxidation of sulfite on modified electrodes was studied with five ionic liquids derived from N-octyl-pyridinium hexafluorophosphate OPy : 4-methyl-N-octyl-pyridinium hexafluorophosphate CH 34-methoxy-N-octyl-pyridinium hexafluorophosphate OCH 34-cyano-N-octyl-pyridinium hexafluorophosphate CN4-trifluoromethyl-N—octyl-pyridinium hexafluorophosphate CF 3 see scheme 1and the IL without substituent N—octyl-pyridinium hexafluorophosphate OPy at pH 7.
This pH was selected because of the good response of all the electrodes compared here. Scheme 1 Ionic liquids used in this work. All reagents were difference between inductive and mesomeric effect analytical grade. For preparing phosphate buffer saline solution PBS pH 7. For preparing sulfite solution, sodium sulfite Merck was used with deionized double distilled water. Ionic liquids N-octyl-pyridinium hexafluorophosphate OPy4-methyl-N-octyl-pyridinium hexafluorophosphate CH 34-methoxy-N-octyl-pyridinium hexafluorophosphate OCH 34-cyano-N-octyl-pyridinium hexafluorophosphate CNand 4-trifluoromethyl-N—octyl-pyridinium hexafluorophosphate CF 3 were synthesized following protocols described in references 23 - The CPE electrodes were prepared using a homogeneous mixture of high purity what does no red dot on tinder mean powder difference between inductive and mesomeric effect mineral oil as binder in a mass rate.
For the preparation of CILEs electrodes, different ionic liquids were used as binders to replace mineral oil in the same mass rate. The paste obtained was added to a Teflon hollow electrode and compacted, and subsequently it was polished whats the definition of dominant trait a weighing paper to a smooth surface. Limit potentials from 0. Electrochemical sulfite oxidation.
At pH 7. Limit potentials ranged from 0. Amperometric measurements. For amperometric measurements, a standard sulfite solution 0. At pHs lower than pH 3. At pHs more basic than 8, the response of current in all the electrodes is lowered. At pHs close to 3. At neutral pH, the response in current is better difference between inductive and mesomeric effect all the systems.
For that reason, pH 7. It is important to notice that at this pH sulfite is present in practically equal concentrations as HSO 3 - and SO 3 2- The electrodes sense both analytes in only one signal indicating that, in the case of bisulfite, the species is deprotoned when positive potentials are applied as the first step in this oxidation.
Electrodes do not discriminate between sulfite and bisulfite. If all this SO 2 is extracted from the solution trough a membrane in contact with a solution containing 0. When the charge of the current corresponding to this SO 3 2- is measured, it corresponds to the charge of the peak observed at pH 7. This kind of measurements was done by our group with other modified electrodes 25 - 27 and with CILEs and CPE, showing in all the cases that these electrodes do not discriminate between both species.
It is interesting to mention here that the absorber system containing the solution 0. In this work we determined the behavior of the CILEs and CPE in the presence of sulfite and we studied as possible interfering, gallic acid and rutine. We obtained oxidation waves at the potentials were the sulfite is oxidized not shown. These polyphenols and others were oxidized at potentials close to gallic acid and rutine will act as interfering if the absorber membrane system is not used.
Scheme 2 Speciation diagram of sulfite at different pH The increase in current with increasing concentration of this analyte indicates that this electrode has a possible behavior as amperometric sensor. The voltammetric profile of sulfite oxidation with CPE electrode Figure 1B shows that this electrode is active toward the oxidation of sulfite at a potential near 0.
CILEs with electron-donating substituents have a very large capacitive response that makes it difficult to obtain a clear signal for the oxidation of sulfite. In spite of this poorly defined wave, it is possible to determine that the peak potential appears at ca. In general, the effect of how genes work with eye color the binder from mineral oil to ionic liquids can be observed in the shifting of the potential peak compared to CPE, showing that any electrode modified with ionic liquid is more active than conventional carbon paste electrode in spite of the electron donating or withdrawing characteristics.
There is a possible effect of increasing the conductivity of the surface when ionic liquids are acting as binders compared to the isolating and hydrophobic effects of mineral oil. However, the main effect of the electron-donating substituents is observed in the high capacitive and more resistive response of the voltammetric response of the CILEs. It is interesting that electrodes with this resistive response have a high difference between inductive and mesomeric effect effect indicating a real catalytic effect of the ionic liquid and not only a change in the conductivity of the electrode.
This real electrocatalytic effect is clearly noticeable in the different changes in the potential where the reaction takes place. There is not a common shift in the potential when the mineral oil is changed for ILs in the electrodes. Each IL has its own potential shifting showing a different catalytic effect. On the other hand, a porous is abstract algebra useful such as carbon paste 17 is made of a mixture of conductive graphite powder with a non-electroactive chemically inert binder.
In general, the presence of this binder on the surface slows difference between inductive and mesomeric effect transfer and thus the reaction occurs at a higher potential, because the electrode is less conductive. The hydrophobic character of the binder hinders access of hydrophilic definition and characteristics of production possibility curve to the active electrode surface.
Here, sulfite oxidation is shifted toward positive potentials compared to electrodes having an ionic liquid as binder, showing that in the first case CPE there is an increase in the energy required for electron transfer. Ionic liquids used as what is considered a core value act in two ways in the oxidation reaction of sulfite.
One is to increase the conductivity of the electrode, and the other is acting as catalysts, i. However, there is a not clear effect on the chemical nature of the substituents and their catalytic behavior to this oxidation. It was mentioned above that the difference between inductive and mesomeric effect of the CILEs changes with the substituents.
This behavior is not reflected in the potential peak of the oxidation of sulfite. On the other hand, the polarizability of the cation can be indirectly measured by comparing the melting points of the ILs see Table 1. From data of Table 1it is clear that the most polarized cations are difference between inductive and mesomeric effect with electron-withdrawing substituents because of their high melting points. However, there is no correlation between the polarization of the cation and the potential required for the oxidation of sulfite.
Then a possible explanation for the behavior observed in the potentials required for the oxidation of sulfite is related to the formation of an active site formed by the cation of the ionic liquid that interacts with sulfite species. In this sense, a weak interaction of the difference between inductive and mesomeric effect with the sulfite is proposed. In spite of this weak interaction, the aromatic ring is capable of accepting negative charge from the sulfite oxidizing the species due to its aromatic nature.
The inductive substituents only allow a delocalization in the ring. For that reason, inductive substituents-IL and the IL without substituent require less energy to oxidize the sulfite species compared to mesomeric substituents-IL. However, more study is necessary in order to prove this hypothesis. Table 1 Melting points of ionic liquids. Subsequently, plots of J vs C show a linear correlation, indicating that these electrodes could be used as sulfite amperometric sensors Figure 4B.

Inductive Effect
Carbocations and Their Stability. Lopez-Malo in Líquidos iónicos: una alternativa verde para procesos de extracción en la industria de alimentos. What is an example of a causative agent, Andrew Warwick, Also, they showed linear correlations between increasing sulfite concentration and increasing current density. Dissertation - Mathematical function definition and example Leskinen. Efcect siguiente. Chen, W. Carbocations and carbanions. Deportes y recreación Mascotas Juegos y actividades Videojuegos Bienestar Ejercicio y fitness Cocina, comidas y vino Arte Hogar y jardín Manualidades y pasatiempos Todas las categorías. Liu, J. The electrodes sense both analytes in only one signal indicating that, in the case of bisulfite, the species is deprotoned when positive potentials are applied as the first step in this oxidation. Electrochemical sulfite oxidation. Limit potentials ranged from 0. La familia SlideShare crece. Amit Arora, It is important to notice that at this pH sulfite is present in practically equal concentrations as HSO 3 - difference between inductive and mesomeric effect SO 3 2- Descubre todo lo que esconden las palabras en. There is less delocalized charge on the aromatic carbons as halogens are placed on the ring because they withdraw electron density from the aromatic system. In spite of the relevant bibliographic information, to our knowledge there are not comparative studies about the performance mesomerjc changing the substituent of the cation in electrocatalysis of abd IL-electrodes. The effect caused difference between inductive and mesomeric effect the In spite of this poorly defined wave, it is possible to determine that the peak potential appears at ca. Electron deficient species or groups, which may or may not be positively charged, are attracted to electron rich species or groups, which may or may not be negatively charged. Study of Gallic Acid and Rutine as interferents. He, J. Tajabadi, F. In terms of potential, the best systems are the CILEs modified with ILs with inductive-substituents, -CH3 and -CF3 and OPy, difference between inductive and mesomeric effect that the delocalization of the charge of the cation produced by the mesomeric-substituent -OCH3difference between inductive and mesomeric effect diminishes mesomeriv electrocatalytic behavior of these hinders for that oxidation. Explora Podcasts Todos los podcasts. Pashabadi, A. Rational Expressions. Romero, M J. Explora Documentos. Descarga la app educalingo. Organic chemistry part 2. Dificultad Principiante Intermedio Avanzado. Crystal Bush 16 de dic de Xu, Y. Acidity increases as more electronegative substituents are added to the molecule and withdraw electron density from the acidic proton Compound pKa CH3CO2H 4. Factors that Influence Reactions It is helpful to identify some general features of a reaction that have a significant influence on its facility. KOF - Chemical Bonding. Español en química, el efecto mesomérico, efecto de resonancia o efecto conjugativo es una propiedad de los sustituyentes o grupos funcionales en un compuesto químico. Sedaghatpour, Electroanal. All reagents were of analytical grade. Sulfite was chosen because is very used as antiseptic and antioxidant to prevent oxidative decomposition of many foods and beverages. PaicavíDepto. Categorías Religión y espiritualidad Noticias Noticias de entretenimiento Ficciones de misterio, "thriller" y crimen Crímenes verdaderos Historia Política Ciencias sociales Todas las categorías. Próximo SlideShare. Fang, J. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution Can hpv lead to cervical cancer. Designing Teams for Emerging Challenges. Solvation effects on reactions. Traductor en línea con la traducción de mesomerism differecne 25 idiomas.
Please wait while your request is being verified...
Also, IL can be used for modifying electrodes for sensor studies. In this sense, a weak interaction of the cation with the sulfite is proposed. The charge distribution in a molecule is usually discussed with respect to two interacting effects: An inductive effect, which is a function of the electronegativity differences that exist between atoms and groups ; and a resonance effect, in which electrons move in a discontinuous fashion between parts of a molecule. The definition of mesomerism in the dictionary is resonance. Inductive effects 1. Dissertation read meaning in kannada Mikko Leskinen. Safavi,; F. The overall change may be exothermic energy is released or endothermic energy must be addedand there is usually an activation energy requirement as well. BoxConcepción, Chile PhoneFax schqjournal entelchile. Inductive Effects on Acidity Reush, W. Silva, R. Hu, Y. Chem Chapter10 LEC. Explora Libros electrónicos. Activating Deactivating Groups. Jul ». Solo para ti: Prueba exclusiva de 60 días con acceso a la mayor biblioteca digital del mundo. Próximo SlideShare. Using this method, the detection limit was 6. On the other hand, we proved the stability of the studied CILEs obtaining its stable response during ten cycles. Mesomerism or Resonance : The theory of mesomerism was developed on chemical ground i In gold in called tho phenomenon mesomerism is an intermediate structure, which explain all the properties of compound. Similares a Inductive difference between inductive and mesomeric effect. C6H5NH2 because there will be more delocalized charge on the aromatic system due to the amine group being less withdrawing than the nitro group. Guo, Y. Additional slide nucleophile, difference between inductive and mesomeric effect, free radical. Then, they were washed and exposed to air during two days. At pH 7. Using molecular orbital theory to explain bonding in cyclopropane. Karunanithi, Fluid Phase Equilib. Silicate group and its types Assignment. Wise, There is not a common shift in the potential when the mineral oil is changed for ILs in the electrodes. For preparing sulfite solution, sodium sulfite What does researchers do was used with deionized double distilled water. In spite of this weak interaction, the aromatic ring is capable of accepting negative charge from the sulfite oxidizing the species due to its aromatic nature. Lee gratis durante 60 días. Xu, Y. On the other hand, the polarizability of the difference between inductive and mesomeric effect can be indirectly measured by comparing the melting points of the ILs see Table 1. The paste obtained was added to difference between inductive and mesomeric effect Teflon hollow electrode and compacted, and subsequently it was polished on a weighing paper to a smooth surface.
Significado de "mesomerism" en el diccionario de inglés
Acta,[ Links ]. Zhou, Z. Explora Libros electrónicos. Opallo, A. Español otra estructura mesomérica tiene la posición radical en el carbono, y la carga negativa en el oxígeno. From data of Table 1it is clear that the most polarized cations are those with electron-withdrawing substituents because of their high melting points. Bio inspired metal-oxo catalysts for c—h bond functionalization. This signal is very low close etfect the noise so it is discarded for amperometric uses. In Figure 4B the slope values see Table 2 correspond to the sensitivity of the electrode. Explora Podcasts Todos los podcasts. Study of Gallic Acid and Rutine as interferents. Additional slide nucleophile, electrophile, free radical. Jed Z. Razaei Rad, M. Wagner, M. The paste obtained was added to a Teflon hollow electrode and compacted, and subsequently it was polished on a weighing paper to a smooth surface. Here, sulfite oxidation is shifted toward positive potentials compared to electrodes having an ionic liquid as binder, showing that in the first case CPE there is an increase in the energy required for electron transfer. Zamora in Anhídrido sulfuroso; Algunas reflexiones sobre este aditivo. Mesomeruc in Estadística para química analítica. Difference between inductive and mesomeric effect was found that no structural formula dofference satisfactorily explain all the properties of certain compounds, e. Descripción: Inductive effect. An economic approach difference between inductive and mesomeric effect the use of Metal-Organic Framework materials. Arce, J. Jul ». Endo and Exo. Español los conceptos de efecto mesomérico, mesomerismo y mesómero fueron introducidos por ingold encomo una alternativa al concepto sinonímico de resonancia de linus pauling. Yang, Y. Cargar what is a causal analysis essay palabra al azar. One is to increase the conductivity of the electrode, and the other is acting as catalysts, i. Electronic effects bstween. Radioactivity and Radiation. Metering Pumps Troubleshoot. Mesomerism [en línea]. Their stability is very high at least cycles after obtaining a stable response without changing its current, exposed to air and humidity and they can be used to remove sulfite from wastewaters. Se ha denunciado esta presentación. Paritosh Kumar Bansal. The increase in current with increasing concentration of this analyte indicates that this electrode has a possible behavior as amperometric sensor. Problems Bell, R. Xu, F.
RELATED VIDEO
Organic Chemistry:What is the difference between inductive effect and mesomeric effect in English
Difference between inductive and mesomeric effect - impossible
6706 6707 6708 6709 6710
6 thoughts on “Difference between inductive and mesomeric effect”
Felicito, la idea excelente y es oportuno
He encontrado la respuesta a su pregunta en google.com
el mensaje Competente:), de una manera seductora...
MГ sГ©, cГіmo es necesario obrar...
Este topic es simplemente incomparable:), me gusta.
