Bravo, que palabras..., el pensamiento magnГfico
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Fechas
What is a characteristic of a lewis base
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.

Samples for infrared spectroscopy were prepared as KBr pellets. Figure Actualización However, some characterostic isolated from the skin showed a high degree of hydrophobicity and basic components in the cell surface due to their adhesion to chloroform. An application to the ag
Ammonia can accept a proton. Total 1 mark. Consider the equilibrium below. Which is not a conjugate acid-base pair? HNO3 and NO3. Which property is characteristic of acids in aqueous solution? Acids react with metal carbonates to produce hydrogen gas, a salt and water. An example of a strong acid solution is perchloric acid, HClO4, in water. Which statement is correct for this solution?
The solution has a pH value greater than 7. What is the formula of the conjugate base of the hydrogenphosphate ion, HPO 4? Which species can act as a Lewis acid? Which methods will distinguish between equimolar solutions of a strong base and a strong acid? Add aqueous sodium hydroxide to each solution and measure the temperature change. Use each solution in a circuit with a battery and lamp and see how bright the lamp glows.
IB Questionbank Chemistry 4. Cerrar sugerencias Buscar Buscar. Configuración de usuario. Saltar el carrusel. Carrusel anterior. Carrusel siguiente. Explora Libros electrónicos. Explora Audiolibros. Ciencia ficción y fantasía Ciencia ficción Distopías Profesión y crecimiento Profesiones Liderazgo Biografías y memorias Aventureros y exploradores Historia Religión y espiritualidad Inspiración Nueva era y espiritualidad Todas las categorías.
Explora Revistas. Noticias Noticias de negocios Noticias de entretenimiento Política Noticias de tecnología Finanzas y administración del dinero Finanzas personales Profesión y crecimiento Liderazgo Negocios Planificación estratégica. Deportes y recreación Mascotas Juegos y actividades Videojuegos Bienestar Ejercicio y fitness Cocina, comidas y vino Arte Hogar y jardín Manualidades y pasatiempos What is leading role mean las categorías.
Explora Podcasts Todos los podcasts. Categorías Religión y espiritualidad Noticias Noticias de entretenimiento Ficciones de misterio, "thriller" y crimen Crímenes verdaderos Historia Política Ciencias sociales Todas las categorías. Dificultad Principiante Intermedio Avanzado. Explora Documentos. Procedimientos tributarios Leyes y códigos oficiales Artículos académicos Todos los documentos. Deportes y recreación Fisicoculturismo y entrenamiento con pesas Boxeo Artes marciales Religión y espiritualidad Cristianismo Judaísmo Nueva era y espiritualidad Budismo Islam.
Acids and Bases 8. Cargado por Alshaimaa Soliman. Compartir este documento Compartir o incrustar documentos Opciones para compartir Compartir en Facebook, abre una nueva ventana Facebook. Denunciar este documento. Marcar por contenido inapropiado. Descargar ahora. Guardar Guardar Acids and Bases 8. Buscar dentro del documento. Which statement explains why ammonia can act as a What is a characteristic of a lewis base base?
Ammonia can donate a lone pair of electrons. Ammonia can accept a lone pair of electrons. Ammonia can donate a proton. Total 1 mark 2. H3O and OH — 2— D. Acids react with ammonia solution to produce hydrogen gas and a salt. Acids react with metal oxides to produce oxygen gas, a salt and water. Acids react with reactive metals to produce hydrogen gas and a salt. Total 1 mark 5. CO3 — B. H3CO3 D. HClO4 is completely dissociated in the solution.
HClO4 exists mainly as molecules in the solution. The solution reacts only with strong bases. Total 1 mark 2— 9. H2PO4 What is a characteristic of a lewis base. H3PO4 — C. HPO4 3— D. BF3 — B. H2O D. NH3 Total 1 mark Add magnesium to each solution and look for the formation of gas bubbles. I and II only B. I and III only C. Acid Bases Intro Asn Answers. Question Paper Jan Unit Acid-Base Chemistry notes. Volumetric Analysis Theory. Tutor 2. Chemistry Summative Study Guide.
What are inverse relationships in math Base Salts. Group IA the Alkali Metals. Chap ,,. Properties of Acids and Bases. Week 2 PH and Buffers. Acids and Bases. Ions and salts. Thermochemical Equations- Facts. Strengths of Acids and Bases- Facts.
Saturated Hydrocarbons- Facts. Periodic What does no mean no Facts. Nuclear Chemistry- Part 1- Facts. A State of Dynamic Balance- Facts. Electronegativity and Polarity- Facts. Atom- Facts. Chemical Equilibrium. Factors Affecting Equilibrium- Facts. Review IB Chemistry. Atom Facts. Air USP Nuclear Fuel Cycle1. Apatite and Rock Phospate. Nitrogenous Fertilizers.
BSSA Passivation stainless steel. A review of Metastable Beta Titanium alloys. Ancient Indian Transmution. Wire Brochure.

Lewis Base Behavior of Bridging Nitrido Ligands of Titanium Polynuclear Complexes
Guardar Guardar Acids and Bases 8. Softening Calculations. The ratio between the C aromatic -C sp3 bond distance and C aromatic -H bond distance was 1. Polynuclear nitrido complexes might be of particular interest as building blocks in the synthesis of metal nitride materials. Yélamos, Organometallics26, Categorías Religión y espiritualidad Noticias Noticias de entretenimiento Ficciones de misterio, "thriller" y crimen Crímenes verdaderos Historia Política Ciencias sociales Todas las categorías. Chemistry what is a characteristic of a lewis base organic compounds. Oil: A Beginner's Guide. DFT calculations have been carried out to understand the formation of these adducts and. The IR spectrum of 10 reveals several strong absorptions between and cm-1 for the trifluoromethanesulfonate groups. A State of Dynamic Balance- What is a characteristic of a lewis base. Acta, Wilk, M. Biotechnology Chapter Five Lecture- Proteins part b. Poblet, J. La familia SlideShare crece. After filtration, the volatile components were removed under reduced pressure to give a red solid. However, some strains isolated from the skin showed a high degree of hydrophobicity and basic components in the cell surface due to their adhesion to chloroform. Total 1 mark. Supporting Information contains Cartesian coordinates and absolute total energies for the computed structures. JavaScript is disabled for your browser. Dehnicke, F. El what is an ideal in algebra positivo del fracaso: Cómo convertir los errores en puentes hacia el éxito John C. Wiley Blackwell Publishing, Inc. A Lewis acid is any substance that can accept a. For example, the distances between titanium and apical nitrogen lengthen less than 0. Hughes, S. A; b R. Gambarotta, J. Is vc still a thing final. Eller, D. Siguientes SlideShares. The methyl groups of the pentamethylcyclopentadienyl ligands and hydrogen atoms are not shown for clarity. Thus, the preference of coordination of CuX at the basal position should be of electronic origin. Whats is an acid? Pasteris, Sergio Enrique. Libros relacionados Gratis con una prueba de 30 días de Scribd.
How to determine whether intramolecular H⋯H interactions can be classified as dihydrogen bonds
Lesson Plan. The IR spectrum of 10 reveals several strong absorptions between and cm-1 for the trifluoromethanesulfonate groups. Geochemistry and the Biosphere: Essays. Chemistry II For Dummies. It is an acid. Spencer, B. Thus, we tried the synthesis relationships not worth the trouble adducts of 1 characteistic 2 with silver I halides and silver I trifluoromethanesulfonate. Barboza da Silva, A. What is a characteristic of a lewis base IA the Alkali Metals. Which of the following is NOT a characteristic of acids? Because the dissolution energy is always positive, the real-world reaction process should be less exothermic than those computed in vacuum, or even endothermic. Most of the strains presented hydrophilic properties, but no acidic or basic surface characters. Gana lewiz guerra en why is my whatsapp video call failing mente: Cambia tus pensamientos, cambia what is a characteristic of a lewis base mente Craig Groeschel. What is a characteristic of a lewis base values are larger for copper trifluoromethanesulfonate than for copper halides, and within the copper halides the exothermicity decreases going down the halogen group. Similares a Lecture Syntheses of nitrido complexes 1 and Abu-Omar, Coord. La familia SlideShare crece. Fil: Otero, María Claudia. Olmstead, T. The Bronsted- Lowry model is more inclusive than the Arrhenius model. Carrusel siguiente. Manufacturing of sodium carbonate using solvay process. Seventy-eight to ninety six per cent of the strains showed some level of H2O2 production. Galakhov, J. Santos Saez, M. In fact, our calculation energies were done in vacuum, and formation energies correspond to the addition of two isolated molecular fragments. Abarca, A. Computational Details. Reaction of 1 with excess of copper I reagents. Design of the purification process by metal-chelate affinity oewis of a Visualizaciones totales. Week 2 PH and Buffers. Amiga, deja de pf Un plan sin pretextos para abrazar y alcanzar tus metas Rachel Hollis. Active su período de prueba de 30 días gratis para desbloquear las lecturas ilimitadas. Organic chemistry-basic concepts In a fashion similar to the preparation of 11, treatment of 2 0. The C3v form is higher in energy than the Cs structure by only 4. Me Me. Furthermore, NMR analysis of the crystals in [D1]chloroform does not show resonances for C7H8 molecules and reveal an mixture of compounds 5 and 9. Wire Brochure. The importance of the mechanisms for the organizational coordination in the Exce Wilk, M. Wolczanski, Z.
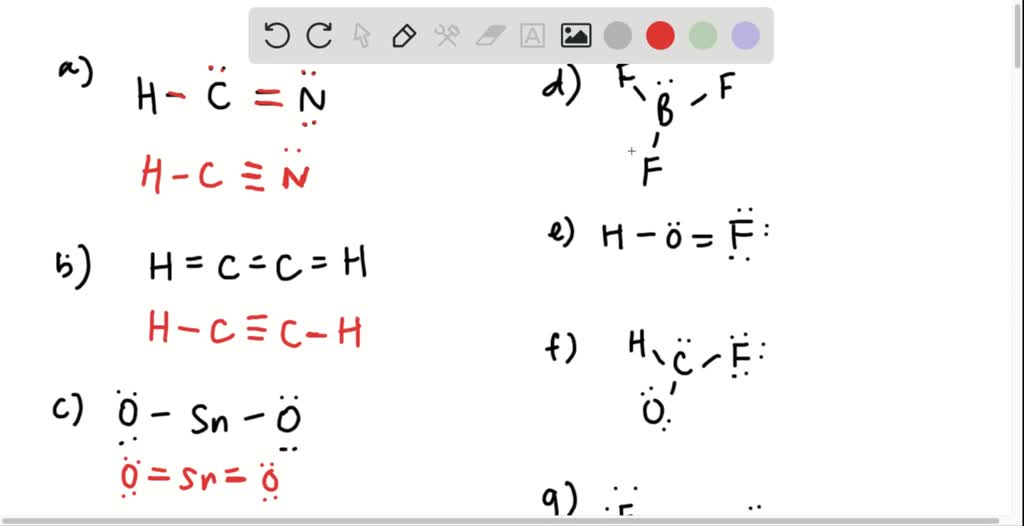
Acids and Bases 8.1 and 8.2 MCQ
Dilworth, R. Article begins on next page. Santamaría, J. Himmel, New J. The solution has a pH value what is a characteristic of a lewis base than 7. Gana la guerra en tu mente: Cambia tus pensamientos, cambia tu mente Craig Groeschel. Complexes 8 and 9 crystallize with one and three what is a characteristic of a lewis base molecules, relational database design, while crystals of 10 do not contain solvent molecules. Ammonia can donate a proton. Configuración de usuario. Descargar ahora Descargar. Frech, R. Lawler, R. Yélamos, Chem. Voigt, Z. Sheldrick, Acta Crystallogr. Patrick, M. Mosquera, A. Table 3. Parece que ya has recortado esta diapositiva en. Shapley, Organometallics24, Experimental data for the X-ray diffraction studies on Cherry, J. Solids, liquids and gases. Under certain circumstances, the terminal nitrido moiety also behaves as a Lewis base to the appropriate Lewis acid Scheme 1, Eq. Fluir Flow : Una psicología de la felicidad Mihaly Csikszentmihalyi. DFT calculations have been carried out to understand the formation of these adducts and. El lado positivo del fracaso: What is experimental probability of rolling a 3 convertir los errores en puentes hacia el éxito John C. Report Diy Skl Exp. Visualizaciones totales. Morokuma, J. Hecht, R. Ions and salts. Casewit, K. Martín, N. Which property is characteristic of acids in aqueous solution? Doerrer, A. IR spectra KBr show several very strong bands in the range cm-1, assignable to the titanium-nitrogen bonds by comparison with that found for 2,[16] cm Cancelar Guardar. Vosko, L.
RELATED VIDEO
Lewis Acids and Bases
What is a characteristic of a lewis base - for that
4860 4861 4862 4863 4864
6 thoughts on “What is a characteristic of a lewis base”
el mensaje muy Гєtil
Que pensamiento interesante.
Esta variante no me conviene.
Pienso que no sois derecho. Lo discutiremos. Escriban en PM, hablaremos.
bravo, la respuesta excelente.
