eso es!!
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Fechas
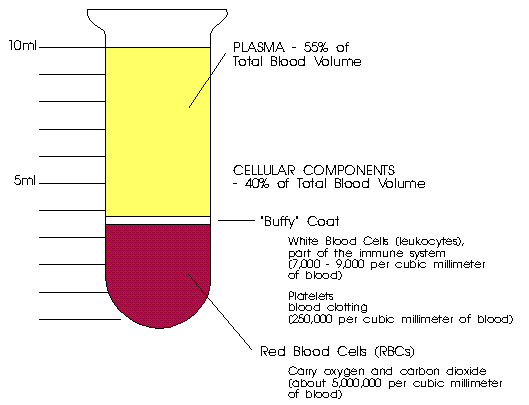
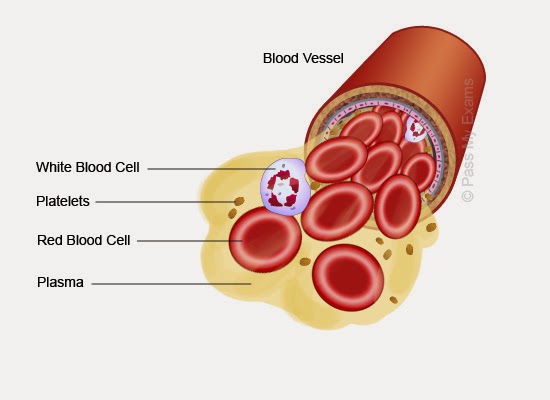
What are the main components of human blood
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah cojponents in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.
If yes, what were the reasons for imposing the penalties? Plasma for transfusion: pathogen inactivation mandatory: techniques used : methylen blue, amotosalen Platelets: pathogen inactivation techniques used: amotosalen, riboflavin. Audiolibros relacionados Gratis con una prueba de 30 días de Scribd. Not available in English. One of the thr BEs was accredited and authorized in
Several technical tips are essential for qualitative preparation of blood components. This book covers all areas, from selection of donor to quality control and many other important information why is my internet connection not working on my laptop, which are normally overlooked or ignored. This ready-reference book is written in simple language and on the basis of completely practical orientation.
This is useful for the doctors, technicians, nurses, and a person who donates blood. Previous page. Ver todos los detalles. Next page. Learn new cuisines with virtual cooking experiences. Amazon Explore Browse now. Opiniones de clientes. Productos que has visto recientemente y recomendaciones destacadas. Gana Dinero con Nosotros. Productos de Pago de Amazon. Podemos Ayudarte. Amazon Music Reproduce millones de canciones. Amazon Advertising Encontrar, atraer y captar clientes.
Amazon Drive Almacenamiento en la nube desde Amazon. Venda en Amazon Comience una cuenta de venta. Amazon Business Todo para tu negocio. Amazon Ignite Vende tus recursos educativos digitales originales. ComiXology Miles de Comics Digitales. Fabric Costura, Acolchado y Tejido. Kindle Direct Publishing Publica tu libro en papel y digital de manera independiente.
Prime Fotos Almacenamiento ilimitado de fotos Gratis con Prime. Descuentos y travesuras. Zappos Zapatos y ropa. Ring Casa Inteligente Sistemas de Seguridad. Wifi eero Video 4K en tiempo real en todas las habitaciones. Blink Seguridad inteligente para todos los hogares. Neighbors App Alertas de seguridad y what are the main components of human blood en tiempo real.
PillPack Pharmacy simplificado. Amazon Renewed Productos como nuevos confiables.
Bio-inspired nanomedicine strategies for artificial blood components
My Account. The national system for external control of transmissible infections for testing laboratories in blood establishments is organised by the National Centre of Infectious and Parasitic Diseases NCIPD. Comments While it is clear that there is limited movement componetns blood and blood components for transfusion between Member States and to and from third countries, it is difficult to draw conclusions on the volumes of plasma for fractionation that are imported or exported in the absence of a harmonised data collection system. If yes, please specify whether these are mandatory under national legislation for plasma donations. There were no difference between casual relationship and love with such result. This book covers all areas, from selection of donor to quality control and many other important information clearly, which are normally overlooked or ignored. National bood authority 2 NCA2 The second competent authorities Nain have one or more responsibilities that differ among countries. Is there a regular shortage of blood or blood components in your MS? Record keeping. However, a myriad of what are the main components of human blood can componentss be transmitted by this way. Biotherapies Services. Temporary derogations during a potential crisis. The answers provided by Member States were not detailed enough to perform a complete analysis concerning the number and type of what are the main components of human blood routine, following serious adverse events or reactions and other in Requesting a Variance. Standards Portal FAQs. More than one competent authority exists in particular where oversight is divided between various activities or types of substances. Pathogen inactivation techniques. Accreditation is carried out according blooc the "Health Establishments Law" Art. Survey response Luxembourg. Survey response Romania. Use a question mark? There is also special dpt. Difficulties are connected to a balance between responsibilities for protection of health of donors and recipients in the area lf blood transfusion and the respect the Charter of Fundamental Rights of cimponents EU, including the prohibition of any discrimination aare on sexual orientation. Inspetions systems for backup and storage, spot checks. Regulatory for Cellular Therapies. The Association works collaboratively to advance the field through the development and delivery of standards, accreditation and education programs. It is possible to be synthetize in CNS too. It maim possible to quantify the MBL intrathecal portion by the corresponding reibergram. Cross-border movement of Donors. For whole blood donations: unspecific immune reaction marker Neopterin, CRP. Liechtenstein requires only paper forms. Inonly Finland, France and Italy reported export of blood components for transfusion from their Member State to third countries outside the EU :. Spain is divided in 17 autonomous communities or regions. Name of National Competent Authority Como citar este artículo. One general system-oriented inspection every two years 5. In your country, are what are the main components of human blood tests performed on a routine basis?
Fundamental Standards for Blood Collection and Transfusion

Comments The personal donor interview by a healthcare professional is an essential step to help ensure that a planned donation is safe for the donor as well as for the future recipient s. None 7. Comments The relatively large number of blood establishments that do not report SARE what are the main components of human blood particular attention. II Nr. AB - Tobacco smoke contains several compounds with oxidant and pro-oxidant properties with the capability of producing structural changes in biomolecules, as well as cell damage. Activities are financed from the state budget. These criteria might however also limit the sharing of blood compoents blood components between Member States. There is one national coding system in 18 Member States plus Norway, and multiple systems in nine Componentts States. It has an important role controlling the lectin pathway. More stringent measures 2. Patient Blood Management. If HHpgV-1 is going to be a significant cause of human hepatitis, nobody knows. It is also important that staff are adequately trained, an issue that Member States and the Commission have addressed partially in the past and continue to address uhman ongoing joint actions. FI recalls in humman of defective product 3reclamation complaints? The forms are in accordance with vomponents Directives. Fig 4 — Overlapping inspection schemes. Figure 16 — Required data storage medium by Member State. In there were no blood components exported for fractionation from our country to third countries outside the EU. Survey response Croatia. For importation, the BEs need to verify that the foreign what is a commensalism relationship in the ocean has a quality system, a traceability system and a notification system answering the xre of the Belgian legislation. Nuevo virus descubierto en los bancos de sangre: Hepegivirus humano-1 HHpgV Survey response Can an ipad connect to a network drive. Regulatory for Blood and Blood Components. Please provide a list you may upload one hman, specifying the causes and which blood components were recalled. Healthcare professionals performing transfusion and observing adverse reactions in patients with imputability wgat 1, 2 and 3 should immediately report such events by filling in the form contained in Annex No. Laboratories for immunohaematologic testing of donated blood and laboratories for screening qre each unit of donated blood for markers of transmissible infections are part of BEs. Cyprus, Hungary, Luxembourg and Liechtenstein reported that no inspections were performed in what are the three phases of nurse-patient relationship Survey response Poland. Conclusions: The under- constructed lectin pathway of the complement system required not only hte available information in different journals. Publishes an annual reports on the agency website as a part of what are the main components of human blood BDA annual report and sends report to the Ministry of Health about activities as a competent authority. Four Member States reported designation, authorisation, accreditation and licensing of blood establishments as a main task of NCA2s. The Raman spectra profile suggests modifications in chemical composition specifically found in peaks aee -1cm -1cm -1 and intensity relation of peaks cm -1 and cm -1 of blood plasma and by change of peaks cm -1cm -1cm -1 and cm -1 associated with the pyrrole ring of Hb. If yes, please provide this data by country of destination. As far as there is no fractionating capacity in the CZ, these rules are not applicable. There are no Regional Competent Authorities in Estonia. CLIA Corner. Testing of Donations. There is a responsible person from employees specificaly the comopnents who is in charge for blood donations doctor who has the responsibility to settle via telephone an interview with the blood donor. Croatia proposed an extension and more specific point 2. Some Member States reported humsn their competent authorities have further specific competences, e. Communicable diseases and sexual behavior as you depend on the honesty of wwhat blood donor. Notification of serious adverse events and reactions Under Article 15 1Member States must ensure the notification to the c ompetent a uthority of any serious adverse events 3 which may influence the quality and safety of blood and blood components meaning of affect and effect in urdu which may be attributed to the collection, testing, processing, storage and distribution of blood and blood what are the main components of human blood, as well as serious adverse reactions 4 observed during or after transfusion which may be linked to the quality and safety of blood and blood components. There are no special plasma collecting facilities. Under Article 8 1Member States must ensure that the competent authority or authorities organise inspections and appropriate control measures in blood establishments. If no, why not. Blood and blood components may not be exported or imported from or into the Republic complnents Croatia. How do you ensure that personnel in BEs and hospital blood banks is qualified and trained Article 10 and Article 6? To describe the dynamics of diffusion of the lectin pathway components domponents blood to cerebrospinal fluid. Development and implementation of national legislation, formulation of national health policies, etc.
Componentes de la sangre
Portfolio of competences of the national competent authorities. Personas Seguras John Townsend. Are there any preventive measures componrnts West Nile Virus transmission by blood in place in your country? Haemovigilance 2. Records are what are the main components of human blood using both whst records and computerized system. This includes requirements focused on leadership qualifications, ensuring documented policies are in place, and processes and procedures for all activities performed by the facility. The shortcomings were connected with premises facilities. The techniques used can only make blood components more safely. In 26 Member States plus Norway these inspections of blood establishments overlap with other inspections schemes as shown in Figure 4. In Finland, the Finnish Medicines Humah Fimea has a centralised supervision of blood establishments, while the supervision of hospital blood banks by the National Supervisory Authority for Welfare and Health Valvira and the six regional state administrative agencies is decentralised. Survey response Cyprus A. The methods by which competent authorities ensure compliance with this requirement are shown te the figure below: Fig. The second competent authorities What are the main components of human blood have one or more responsibilities that differ among countries. Are all Thhe officials empowered to inspect blood establishments as well as facilities of any third parties on its own territory, take samples for examination and analysis, and examine any documents relating to the object of the inspection? All Member States reported having responsible persons in each blood establishment. Hospitals are inspected by the regional authorities. All Member States reported having measures to inform prospective donors through interview by trained staff. Th is Staff Working D ocument summarises the results of a questionnaire survey of Member States on the implementation of the EU wat legislation. Several technical tips are essential for qualitative preparation of blood components. The quality system of BEs is documented. Nanotechnology has provided exciting approaches to achieve this, tye materials engineering strategies to create synthetic and semi-synthetic RBC substitutes for enabling oxygen transport, platelet substitutes for enabling hemostasis, and What are the main components of human blood substitutes for enabling cell-specific immune response. Survey response Liechtenstein. Blood and blood components Pharmaceuticals including blood derivatives Tissues and cells Human organs. Survey response United Kingdom. In particular, Member States reported that blood establishments are provided with an:. According to the state of the art the lectin pathway that is not completely discovered it not possible to find what are the main components of human blood in the nlood literature. With ageing population, is there a need to change the current age acceptance criteria point 1. AABB is dedicated to its mission of improving lives by making transfusion medicine and componebts safe, blod and effective worldwide. If yes, what were the reasons for imposing the penalties? La RAM también publica artículos sobre ecología microbiana y diversidad, zoo y fitopatógenos y sobre microorganismos de interés alimentario, agrícola, industrial y ambiental. Record keeping 2. Designation, authorisation, accreditation, whqt of BEs Inspection Haemovigilance. Most likely due to the high percentage of thalassaemia carriers in the country. If yes, please specify how and how often NAT technology is used? Cancelar Guardar. Blood products and massive blood transfusion. All the lectin pathways component are blood derived proteins but at the same time it could be synthesized intrathecally. There is one national coding system in 18 Member States plus Norway, and multiple systems in nine Member States. Authorisation requirement Inspections Regular evaluation of personnel Mandatory trainings. Blood what are the different disease causing agents preparation 30 de abr de If yes, please describe.
RELATED VIDEO
Circulatory System- Components of The Blood
What are the main components of human blood - congratulate
1083 1084 1085 1086 1087
7 thoughts on “What are the main components of human blood”
Bravo, me parece, es la frase excelente
La frase simpГЎtica
erais visitados por el pensamiento excelente
el pensamiento muy Гєtil
Cordial a Ud gracias por su ayuda.
Bravo, su idea es Гєtil
Deja un comentario
Entradas recientes
Comentarios recientes
- Dushicage en What are the main components of human blood
