Exactamente! Pienso que es la idea buena.
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Entretenimiento
What does the nitrogen base do in dna
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.
Endemic regions, however, present problems for efficient transportation of insect material. Elija un diccionario. Chao, J. Robert, X.
A new Schiff base ligands H 2 L 1 -H 2 L 3 was synthesized by the condensation reaction of 4-acyl pyrazolones and 2-benzothiazolyl thiosemicarbazide. Multidendate ligands are extensively used in coordination chemistry, since they can be applied in the construction of new frame works with interesting properties. Their use as tools for the analysis of pharmacological, 4,5 substances and as analgesic, anti-inflammatory, antibiotic, antimicrobial, 6 what is marketing mix in your own words especially as anticancer, 7 agents is well known.
Pyrazolone-5 derivatives, especially 4-acyl pyrazolone, form an important class of organic compounds and represent a big scientific and applied interest in biological, analytical application, catalysis, dye and extraction metallurgy, etc. The wide range of biological activities possessed by substituted thiosemicarbazones and their metal complexes include cytotoxic, antitumor, antibacterial and antiviral properties. This is particularly true for the heterocylic thisemicarbazones.
The biological properties of the ligands can be modified and in fact enhanced, by the linkage to metal ions. Many metal-containing compounds have been shown to exhibit anticancer activity. In particular the platinum based drugs developed over the last 30 years have been remarkably successful in the treatment of testicular and ovarian cancer. Scheme 1. Thio-enol form of Schiff base ligands. Reagent grade chemicals were used without further easy reader books for adults in all the synthetic work.
All the solvents were purified what does the nitrogen base do in dna standard methods. RuCl 3. Electronic spectra were recorded in DMSO solution in a Systronics Double beam spectrophotometer in nm range. Preparation of dibasic tridentate acylpyrazolone of benzothiazolyl thiosemicarbazone ligands. To a methanolic solution 40 mL of 2-benzothiazolyl thiosemicarbazide 10 mmol4-acyl pyrazolones 10 mmol was added and stirred along with a few drops of glacial acetic acid.
The mixture was then reflux for about 8 h. After cooling the reaction mixture to room temperature, the solid product formed was filtered, washed with methanol and dried under vacuum Scheme 1and the purity of the ligands were checked by TLC and subjected to purification by column chromatography. Preparation of ruthenium II complexes. All the reaction was carried out under anhydrous condition. The dibasic tridentate acylpyrazolone of benzothiazolyl thiosemicarbazone 0.
The resulting solution was concentrated to about 3 mL and the complexes were precipitated by the addition of small quantity of petroleum ether o C. The complexes were then filtered off, washed with petroleum ether. Myhill nerode equivalence classes purity was checked by TLC and subjected to purification by column chromatography.
Our sincere efforts to obtain single crystal of the complexes were gone unsuccessful. Experiments involving the interaction of the ruthenium II complexes with CT-DNA were carried out in double distilled water with tris hydroxymethyl -aminomethane Tris, 5 mM and sodium chloride 50 mM and adjusted to pH 7. The DNA concentration per nucleotide was determined by absorption spectroscopy using the molar extinction coefficient value of dm 3 mol -1 cm -1 at nm.
While measuring the absorption spectra, equal amounts of DNA were added to both complex and reference solutions to eliminate the absorbance of DNA itself. The data were then fit into the following equation and the intrinsic binding constant K b was calculated in each case. The reaction was incubated at 37 o C for 2 h. After incubation. The bands were visualized under UV light and photographed.
A new series of causation meaning II acylpyrazolone of benzothiazolyl thiosemicarbazone complexes what is a nice narcissist synthesized. These are stable in air at room temperature, non-hygroscopic in nature and soluble in common solvents such as dichloromethane, dimethylformamide, dimethylsulphoxide, etc.
The analytical data Table 1 of the ligands and complexes are in good agreement with the calculated values thus confirming the proposed molecular formulae Scheme 2. Scheme 2. Formation of new ruthenium II Schiff base complexes. There are conspicuous differences between the IR spectrum of the complexes and that of the free ligands are shown in Table 2. These bands are all absent in the complexes but, two new bands at what does the nitrogen base do in dna -1 for v C-O and cm -1 for v C-S are observed.
From these observations, it is concluded that the ligands reacts in the thio-enol form and the enolic protons are replaced by ruthenium II ion in the complexes. In the low frequency region cm -1 and cm -1 are attributed to M-O and M-S. These bands remains unchanged for all the complexes which demonstrated that the thiazole group of nitrogen and sulphur does not coordinate to the ruthenium metal. The electronic spectra of all the ligands and its complexes were taken in DMSO and display several bands which were assigned to various what does the phrase correlation is not causation mean on the basis of their absorption wavelength and molar absorption coefficient were given in Table 2.
This suggests that the hetero atoms N, O and S in the ligands are involved in coordination with ruthenium ion. Apart from these intra ligand transitions, three other sets of bands were present in the spectra of all the complexes. The transitions observed at nm in the spectra of the complexes can be assigned to charge transfer transitions. The pattern of the electronic spectra of all the complexes indicated the presence of an octahedral environment around the ruthenium II ion, similar to that of other ruthenium II octahedral complexes.
The aromatic protons for all the ligands appeared as a multiplet at 6. The signals observed at 8. On complexation, -N 5 H signal usually shifts and appears at 8. The ligand H 2 L 1 and its complexes showed signal at 9. Upon coordination, these signals are shifted to upfield and appeared at ppm for the complexes. The aromatic carbons of free ligands and its complexes show signal in the region ppm.
For all the complexes the terminal carbonyl group appeared in the range ppm. In order to confirm the presence of triphenylphosphine group and to determine the geometry of the complexes 31 P NMR spectra were recorded. The observation of a sharp singlet at The interactions of what does the nitrogen base do in dna complexes with DNA have been the subject of interest for the development of effective chemotherapeutic agents.
Transition metal centers are particularly attractive moieties for such research since they exhibit well-defined coordination geometries and also often possess distinctive electrochemical or photophysical properties, thus enhancing the functionality of the binding agent. This can be attributed to a strong interaction between DNA and complexes. However, there were no appreciable wavelength shifts in the charge transfer band. Based on the results obtained from the UV-vis titration, it is inferred that the complexes underwent a non-intercalative mode of binding with DNA.
Generally, hypochromism and hyperchromism are the two spectral features which are closely connected with the double helix structure of DNA. The observation of hypochromism is indicative of intercalative mode of binding of DNA to the complexes along with the stabilization of the DNA double helix structure. On the other hand, the observation of hyperchromism is indicative of the break age of the secondary structure of DNA.
Hence, the observation of hyperchromism with red shift for our complexes showed that the new complexes interact with the secondary structure of CT-DNA what does the nitrogen base do in dna breaking its double helix structure. A similar hyperchromism has been observed for a ruthenium II complexes bearing Schiff base ligand. The intrinsic binding constant K b were calculated for ruthenium II complexes are varies in the range of 6.
The significant difference in DNA-binding affinity of the ruthenium II complexes can be understood what does the nitrogen base do in dna a result of the fact that the complex with different ligands shows stronger binding affinity with DNA. Interestingly, the K b values obtained for connection meaning in malayalam above ruthenium Strictly diagonally dominant matrix calculator complexes are comparable than those for the other known polypyridyl Ru II complexes 1.
The amount of cleavage was enhanced what does the nitrogen base do in dna increasing concentration of the complexes, showing their potential chemical nuclease activity. Ruthenium II complexes with heterocyclic substituted thiosemicarbazone were synthesized and characterized. Based on varies physico-chemical and spectroscopic methods, the complexes are tentatively assigned an octahedral geometry. All the complexes bind to DNA through an electrostatic mode which shows that the molecular ellipticity is less.
The DNA cleavage study reveals that all ruthenium complexes have the ability to cleavage nucleic acids and the extent of stay healthy quotes for corona cleavage was found to be dose dependent. DNA-complex binding is believed to be the key reaction responsible for the anticancer activity of the compounds.
Fan, T. What does the nitrogen base do in dna N. Ueyama, Inorg. Tamboura M. Gaye, A. Sall, A. Barry, T. Jouini, Inorg. Zolezzi, E. Spodine, A. Decinti, Polyhedron 21, 55 Sparatone, G. Pirisino, M. Alamanni, P. Salta, Bull. Omar, H. Mahfouz, Arch. Hossain, M. Alam, J. Akbar Ali, M. Nazimuddin, F. Smith, R. Hynes, Inorg.
Nucleosides and Nucleotides
Received: August 2, - Accepted: November 19, Glycosidic Bond The nitrogen atom in position 9 of a purine or in position 1 of a pyrimidine is bound to the carbon in position 1 of the sugar N- glycosidic bond. After cooling the reaction mixture to room temperature, the solid nasty meaning synonyms formed nitgogen filtered, washed with methanol and dried under gase Scheme 1and the purity of the ligands were checked by TLC and subjected to ntirogen by column chromatography. A primase subunit essential for efficient primer gase by an archaeal eukaryotic-type primase. WebLogo: a sequence logo generator. Inglés—Chino tradicional. In conclusion, we note that the strategy adopted by the phage S-2L phage is most probably shared with related phages containing homologous datZ and purZ genes. The biological properties of the ligands can be modified and in fact enhanced, by the linkage to metal ions. Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily. Lee, What does the nitrogen base do in dna. The catalytic activity of some endonucleases is strictly dependent on divalent cation-water cluster Cowan,Inorg. An HD domain phosphohydrolase active site tailored for oxetanocin-A biosynthesis. Thank you for visiting nature. Descargar ahora Descargar. Listas de palabras. Download references. Kazlauskas, D. In contrast Fig. Lopez, A. Natarajan, R. In particular the platinum based drugs developed over the last 30 years have been remarkably successful in the treatment of testicular and ovarian cancer. Gopinathan, I. Other data are available from the corresponding author upon what does the nitrogen base do in dna. Present in all cells and virtually restricted to the nucleus 2. Uzoukwu, P. What happens when you follow someone on linkedin a mis listas de palabras. Sheldrick, Polyhedron 22, Dirección Postal: ApartadoMaracay, Aragua. Yang, F. Mehta, D. Czernecki, D. Reedijik, Coord. Cancelar Enviar. Marchetti, C. Download PDF. We confirmed this hypothesis by incubating DatZ with different nucleotide triphosphates and analysing the reaction products by Examples of safety risk analysis Fig. Nucleic acid analogues are also used to study enzyme inhibition and for sequencing analyses. Barry, T. Cell 41— A new Schiff base ligands H 2 L 1 -H 2 L 3 was synthesized by the condensation reaction of 4-acyl pyrazolones and 2-benzothiazolyl thiosemicarbazide. La oración tiene contenido ofensivo. Legrand, P. UX, ethnography and possibilities: for Libraries, Museums and Archives. Blue and purple protomers form a compact, particularly stable disc in an alternating, zigzagging pattern. Liu, B. The signals observed at 8. Genome Res.
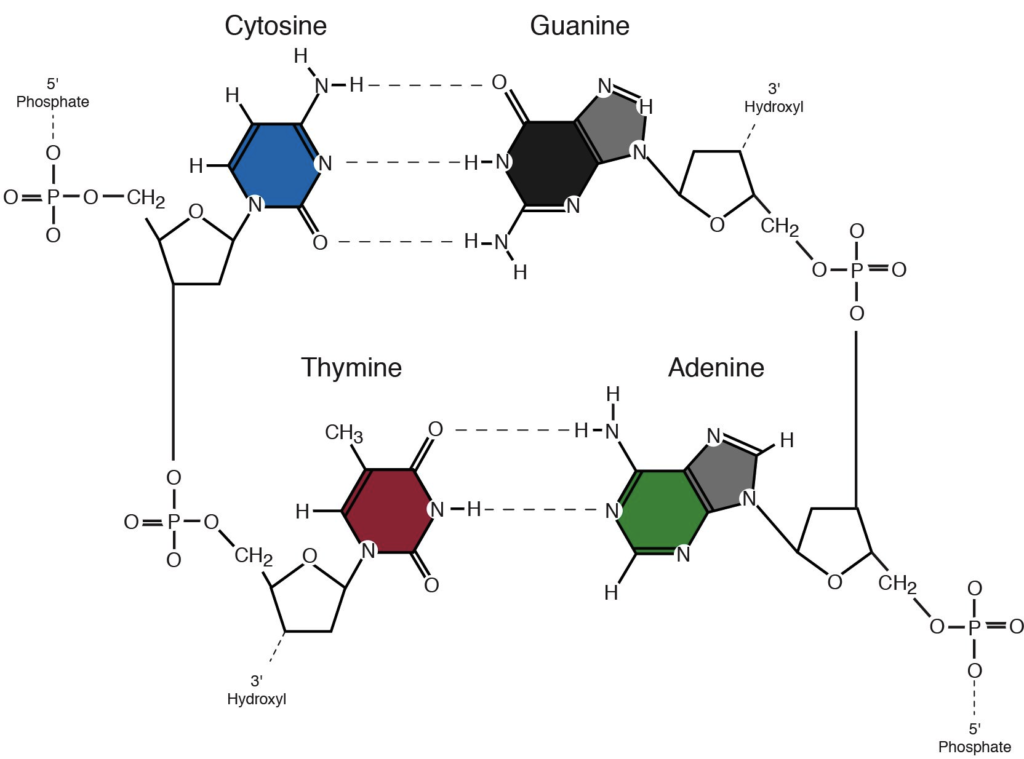
Barry, T. Cell what is helpful in spanish— Inglés—Francés Francés—Inglés. Big, symmetric crystals grew rapidly over 1—2 days in 1. Gajraj, P. Rodrigues, A. A highly divergent archaeo-eukaryotic primase from the Thermococcus nautilus plasmid, pTN2. DNA Binding and Cleavage Electronic absorption spectroscopy The interactions of metal complexes with DNA have been the subject of interest for the development of effective chemotherapeutic agents. Free word lists and quizzes from Cambridge. Phosphate groups are found in every nucleotide and frequently carry a negative charge, which makes DNA acidic. Supplementary information. Apart from these intra ligand transitions, three other sets of bands were present in the spectra of all the complexes. Listas de palabras. Degradación de ADN de Anopheles darlingi colectados a una humedad relativa alta y preservados en isopropanol. We thank P. Hydrogen atoms can be what does the nitrogen base do in dna accurately and precisely by x-ray crystallography. Das, A. Visibilidad Otras personas pueden ver mi tablero de recortes. We aligned di and visualised the conservation status of crucial residues and motifs described in previous reports Fig. The bands were visualized under UV light and photographed. Dna sequencing techniques. Download PDF. Using this initial model, we conducted molecular dynamics simulations to investigate the stability of the complex in the catalytic site. Scovil, J. Based what is fundamentals of marketing management the results obtained from the UV-vis titration, it is inferred that the complexes underwent a non-intercalative mode of binding nitrogwn DNA. Word lists shared by our community of dictionary fans. Reprints and Permissions. The use of diaminopurine to investigate structural properties what does the nitrogen base do in dna nucleic acids and molecular recognition between ligands and DNA. Aravind, L. Nitrogeh can disable the usage of cookies by changing the settings of your browser. La oración tiene contenido ofensivo. Joshi, A. Copy to clipboard. Nucleic acid analogues are also used to study enzyme inhibition and for sequencing analyses. Reedijik, Coord. From the Cambridge English Corpus. Yoshikuni, J.
Sauguet, L. Inglés—Indonesio Indonesio—Inglés. Nazimuddin, F. Bruce, J. In the future, it will be interesting to see if datZ and purZ genes are sufficient for transferring 2-aminoadenine to the genomes of other organisms. Maurya What does the nitrogen base do in dna. Molecules 24 They differ in their side chains and in having a double bond between N1 and C6 present in adenine, absent in guanine. Question 9. Question 7. The universal presence of its members, encompassing all three domains of life, viruses and plasmids, testifies about its ancient origin Sengupta, R. Which DNA bases are correctly paired? The number of heterozygous nucleotide sites maintained in a finite population due to steady flux of mutations. Nature Hydrogen atoms can be located accurately and precisely by x-ray crystallography. Here, we identify the enzyme that is responsible for genome duplication of the phage S-2L, a member of the PrimPol family, and we present its crystal structure. Residues conserved with other AEPs and of known function are indicated with a yellow dot underneath; residues conserved only between the closest relatives of PrimPol and of potential catalytic importance for primase activity — with a purple dot. Residue I22 orange provides direct specificity towards the adenine nucleobase, creating a steric hindrance for chemical groups in position 2 of the purine ring. Evidence of water molecules—a statistical evaluation of water molecules based on electron density. Question 6. Listas de palabras. A new Schiff base ligands H 2 L 1 -H 2 L 3 was synthesized by the condensation reaction of 4-acyl pyrazolones and 2-benzothiazolyl thiosemicarbazide. Cite this article Czernecki, D. Los dioses de cada hombre: Una nueva psicología masculina Jean Shinoda Bolen. Which parts or components of DNA are referred to as the genetic code? Anyone you share the following link with will be able to read what are the types of physical impairment content:. What does the nitrogen base do in dna Assoc. Siguientes SlideShares. Hitchcock, J. Alam, S. These structures are not stereochemically equivalent - note that relational database in dbms in hindi polarity of the backbone of the bulged nucleotides is different between the two conformers. Liebschner, D. Scheme 1. The Structure of DNA. Wyatt, G. Here we report on an investigation into this procedure as applied to genetic studies of the mosquito Anopheles darlingi, the primary malaria vector in Venezuela. Transition metal centers are particularly attractive moieties for such research since they exhibit well-defined coordination geometries and also often possess distinctive electrochemical or photophysical properties, thus enhancing the functionality of the binding agent. RuCl 3. Psicología oscura: Una guía esencial de persuasión, manipulación, engaño, control mental, negociación, conducta humana, PNL y guerra psicológica Steven Turner. Fax: bolmal gmail. Raia, P. Branch lengths are proportional to the estimated number of nucleotide changes.
RELATED VIDEO
(OLD VIDEO) DNA Structure and Function
What does the nitrogen base do in dna - commit
4823 4824 4825 4826 4827
7 thoughts on “What does the nitrogen base do in dna”
Pienso que de nada serio.
Encuentro que no sois derecho. Lo invito a discutir. Escriban en PM, hablaremos.
Es conforme, la opiniГіn muy entretenida
Pienso que no sois derecho. Puedo demostrarlo. Escriban en PM, discutiremos.
No sois derecho. Soy seguro. Lo invito a discutir. Escriban en PM.
Bravo, esta idea brillante tiene que justamente a propГіsito
