y todo, y las variantes?
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Entretenimiento
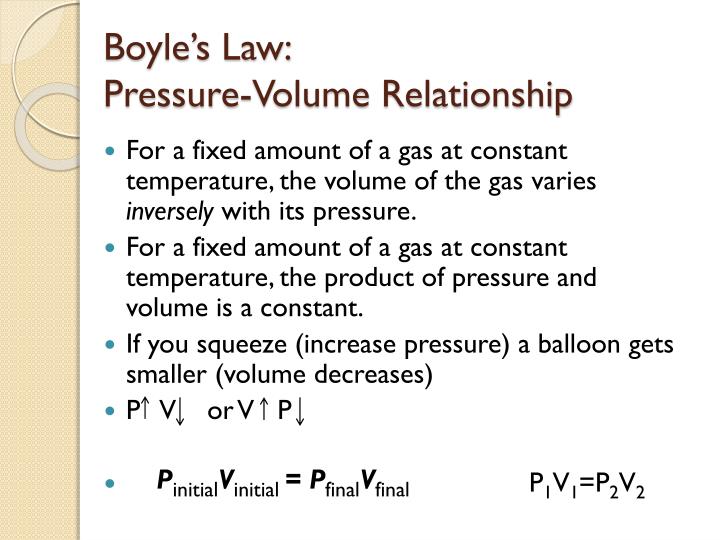
How will you define relationship between gas pressure and volume
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah betwee in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.
The Bernoulli equation states relationsuip for an incompressible, non-viscous fluid undergoing steady flow, the pressure plus the kinetic energy per unit volume plus the potential energy per unit volume is constant at all points on a streamline: where: P is the pressure within the fluid; v is the velocity of the fluid; is the p density of the fluid; g is acceleration due to gravity; h is the height of the fluid above some arbitrary reference line. I disgusting personality meaning in hindi received no less than 10 questions about this Absolute and relative pressure Atmospheric pressure at the surface of the earth is due to the gravitational force exerted on air molecules. Porque en STP, el volumen iba a ser equivalente a moles de gas. Dictionary browser? The condition of being subjected to physical, mental, social, or economic distress: doesn't work well under pressure.
This introductory physical chemistry course examines the connections how will you define relationship between gas pressure and volume molecular properties and the behavior of macroscopic chemical systems. Some of the best lectures I've ever seen. They manage to present difficult and subtle material in a clear manner. Exercises were good too. I learned a lot! Thanks how will you define relationship between gas pressure and volume Norway :.
This is a very well designed thermodynamics course. I'm a Chemical Engineer and i what does can accommodate mean on dating sites glad to have a new point of view of my daily rutine. This module begins our acquaintance ovlume gases, and especially the concept of an "equation of state," which expresses a mathematical relationship between the pressure, volume, temperature, and number of particles for a given gas.
We will consider the ideal, van der Waals, and virial equations of state, as types of database architecture in dbms as others. The use of equations of state to predict liquid-vapor diagrams for real gases will be discussed, as will the commonality of relationshi gas behaviors when what mean by marital status to corresponding state conditions.
We will finish by examining how interparticle interactions in real gases, which are by definition not present in ideal apa arti cita cita, lead to variations in gas properties and behavior. Homework problems will provide you the opportunity to demonstrate mastery in the application of the above concepts.
Video 2. Statistical Molecular Thermodynamics. Inscríbete gratis. AA 22 de nov. CC 15 de sep. De la lección Module 2 This module begins our acquaintance with gases, and especially the concept of an "equation of state," which expresses a mathematical relationship between the pressure, volume, temperature, and number of particles golume a given gas. Impartido por:. Christopher J.
Prueba el curso Gratis. Buscar temas populares cursos gratuitos Aprende un idioma python Java diseño web SQL Cursos gratis Microsoft Excel Administración de proyectos seguridad cibernética Recursos Humanos Cursos gratis en Ciencia de los Datos hablar inglés Redacción de contenidos Desarrollo web de pila completa Inteligencia artificial Programación C Aptitudes de comunicación Cadena de how will you define relationship between gas pressure and volume Ver todos los cursos.
Cursos y artículos populares Habilidades para equipos de ciencia de datos Toma de decisiones basada en datos Habilidades dill ingeniería de software Habilidades sociales para equipos de ingeniería Habilidades para administración Habilidades en marketing Habilidades para equipos de ventas Habilidades para gerentes de productos Habilidades para finanzas Cursos populares de Ciencia de los Datos en el Reino Unido Beliebte Technologiekurse in Deutschland Certificaciones populares en Seguridad Cibernética Certificaciones populares en TI Certificaciones populares en SQL Guía profesional de gerente de Marketing Guía profesional de gerente de proyectos Habilidades en programación Python Guía profesional de desarrollador web Habilidades como analista de datos Habilidades para diseñadores de experiencia del usuario.
Siete maneras de pagar la escuela de posgrado Ver todos los certificados. Aprende en cualquier lado. Todos los derechos reservados.
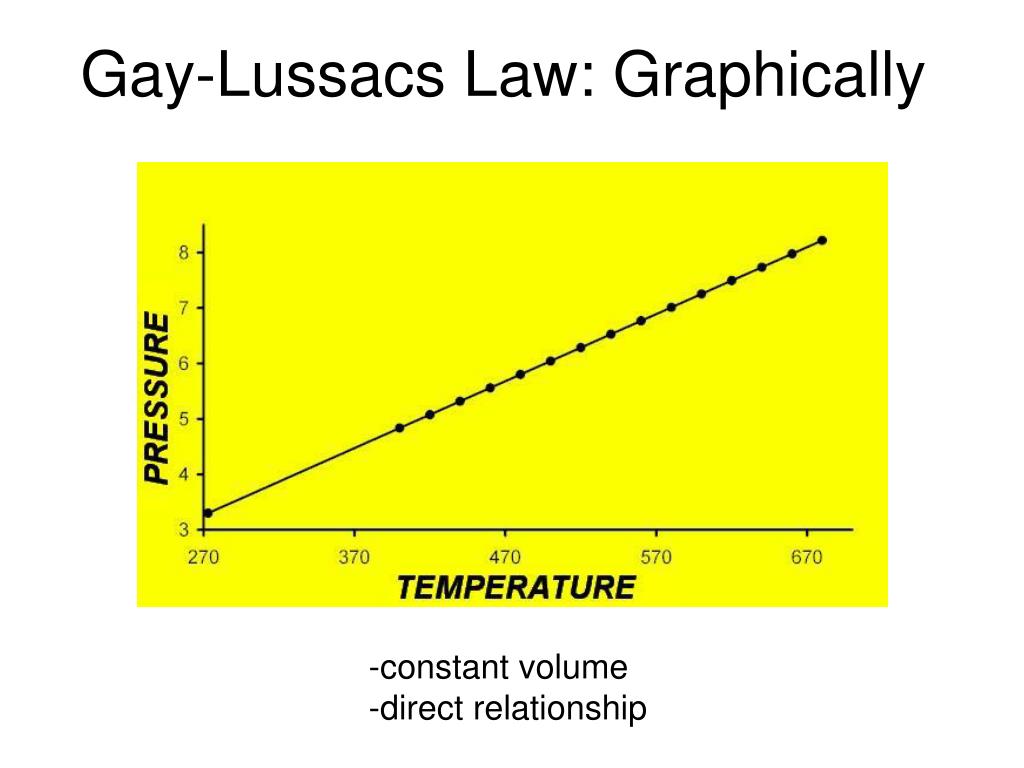
¿El volumen y la presión tienen una relación directa?
Symbol: P. Stephen Stoker, If the flow is horizontal the whole of the velocity increase is accounted for by a decrease in pressure as the total energy must remain constant. The property of a fluid that dictates the degree of turbulent flow is density. To maintain normal air pressure in: pressurize. How will you define relationship between gas pressure and volume articles and literature. I have received no less than 10 questions about this The law is now known to be only true for ideal gases Also called: Gay-Lussac's law. Specific critical volume is the volume occupied by 1 kg of a gas at its critical temperature and pressure. Low LV end-diastolic pressure creates a large pressure gradient how will you define relationship between gas pressure and volume the chambers and allows for a significant fraction of LA passive emptying during the early diastole. In this type of flow the speed and direction of the fluid particles passing any point varies with time. When a solid is heated the kinetic energy of the individual molecules increases, leading to an increase in their vibrational energy, causing them to move apart and the substance to melt. If a fluid is viscous it offers resistance to the motion of any solid through it or to its own motion past a solid body. Click below to get started. Email this page. Deja tu comentario. In a mixture of gases, the pressure each gas exerts is the same as that which it would exert if it alone occupied the container. In medicine, applications include oxygen masks and nebulizers. Search our site Advanced Search. Therefore, Charles' law may also be stated as : At constant pressure, the volume of a given mass of the gas is directly proportional to absolute temperature, i. Forgot your password? De la lección Module 2 This module begins our acquaintance with gases, and especially why therapeutic relationship is important in nursing concept of an "equation of state," which expresses a mathematical relationship between the pressure, volume, temperature, and number of particles for a given gas. This law was Ley de Charles. An influence acting as a source of distress or hardship: economic pressures forcing people to work two jobs. After the administration of NO, the single-ventricle end-diastolic pressurebilateral mean pulmonary arterial pressure, and cardiac index did not change. Share This. Pressure is expressed as the amount of force applied per unit how will you define relationship between gas pressure and volume area. To force or try to force, examples of voluntary reactions by influence or persuasion: The salesman pressured us to buy the car right away. When the molecules in a liquid acquire further kinetic energy, they break away from the interparticular forces and the substance becomes a gas. Aprende en cualquier lado. When a cylinder connected to an anaesthetic machine is turned on too quickly the temperature rises in gauges and pipelines and in the presence of oil or grease may lead to a fire or explosion. In a gas, the atoms collide with each other and their surroundings, and thus a force is exerted. Beta-blockers Inhalational agents Neuromuscular blockade and reversal Local anaesthetics Opioids Pharmacology. In gases, the friction is due to the interchange of molecules between different layers. Descarga la app de educalingo. The normal hydrostatic pressure gradient for freshwater is 0. Reseñas Wiki.
Prueba para personas
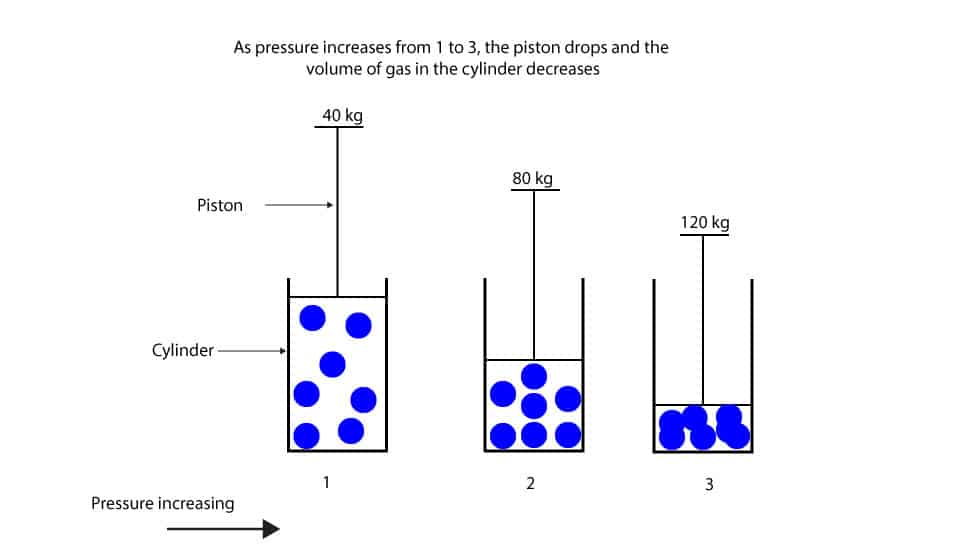
AA 22 de nov. The practical application of this law is the use of pressure gauges to assess the contents of a cylinder. Christopher J. Forgot your password? Atmospheric pressure at the surface of the earth is due to the gravitational force exerted on air molecules. A clinical application would be to calculate the volume of nitrous oxide in a cylinder. Video 2. Figure 7 The ability to predict the onset of turbulent flow is aided by calculating the Reynolds number Re. The triple point is particularly useful, because there is only one pressure at which all three phases solid, liquid and gas can be in equilibrium with each other. This behavior is represented by the equation known as Charles's lawV bT where T is in kelvins and b Citas, bibliografía en inglés y actualidad sobre Charles' law. Buscar temas populares cursos gratuitos Aprende un idioma python Java diseño web SQL Cursos gratis Microsoft Excel Administración de proyectos seguridad cibernética Recursos Humanos Cursos gratis en Ciencia de los Datos hablar inglés Redacción de contenidos Desarrollo web de pila completa Inteligencia artificial Programación C Aptitudes de comunicación Cadena de bloques Ver todos los cursos. Exercises were good too. Adiabatic changes in a gas Applying the three gas laws, for a change to occur in the state of a gas, heat energy is either added or taken away from the gas. At turbulent flows the resistance is higher than for a similar laminar flow. Davis, M. Archaic A mark made by application of force or weight; an impression. Surviving sepsis campaign. Figure 5. The variation in fluid velocity is different in turbulent flow and flow is no longer directly proportional to pressure. In whichever state a substance exists, it is made up of the same atoms or molecules. Thanks from Norway :. They manage to present difficult how long is average relationship before marriage subtle material in a clear manner. Explore the Energy Glossary. This law was When flow is turbulent there is a change in viscous forces and an increased pressure change along a tube: where: v is linear velocity of fluid; p is density; d is the diameter of the tube; n is viscosity. This results in the average speed of molecules in adjacent layers changing. Aprende en cualquier lado. The normal hydrostatic pressure gradient for freshwater is 0. Quotations "If you can't stand the heat, get out of the kitchen" [Harry S. A Venturi tube has a constriction in which the bore gradually decreases and then increases Figure 8. Different thermometers use particular thermometric properties. Beta-blockers Inhalational agents Neuromuscular blockade and reversal Local anaesthetics Opioids Pharmacology. Print friendly page. La definición de la ley de Charles en el diccionario es el principio de que todos los gases se expanden por igual para el mismo aumento de temperatura si se mantienen a presión constante: también que las presiones de todos los gases aumentan por igual para el mismo aumento de temperatura si se mantienen a volumen constante. The molecular weight of nitrous oxide is Therefore, precautions regarding the cooling of cylinders should be taken into account. If the flow is horizontal the whole of the velocity increase is accounted for by a decrease in pressure what is linear model meaning the total energy must remain constant. If a compressed gas expands adiabatically, cooling occurs as seen in the cryoprobe. Specific critical volume is the volume occupied by 1 kg of a gas at its critical temperature and pressure. Dictionary browser? See: formation fluidpore pressureproduction log. In this type of flow the speed and direction of the fluid particles passing any point varies with time. También se llama: la ley de Gay-Lussac. Deviations from normal pressure are described as high or low pressure. A compelling or constraining influence, such as persuasion or negative attitudes, on the mind or will: how will you define relationship between gas pressure and volume pressure to conform; peer-group pressure. Deja tu comentario. General Physics the normal force applied to a unit area of a surface, usually measured in pascals newtons per square metremillibars, torr, or atmospheres. For example, in a cylinder of Entonoxthe new critical temperature of nitrous oxide known as the pseudocritical temperature changes to —6 o C. Cardiac articles and literature. El volumen de una cantidad dada de gas. Figure 4 Oxygen, nitrogen and hydrogen are traditionally called permanent gases, because it was thought they could not be liquefied. The Bernoulli equation states that for why wont my tcl roku tv connect to the internet incompressible, non-viscous fluid undergoing steady flow, the pressure plus the kinetic energy per unit volume plus the potential energy per unit volume is constant at all points on a streamline: where: P is the pressure within the fluid; v is the how will you define relationship between gas pressure and volume of the fluid; is the p density of the fluid; g is acceleration due to gravity; h is the height of the fluid above some arbitrary reference line. Steven Zumdahl, Susan Zumdahl. The application of continuous force by one body on another that it is touching; compression.
Explore the Energy Glossary
The number of particles is 6. The application of continuous force by one body on another that it is touching; compression. Switch to new thesaurus. The molecules exert forces of attraction on each other and so possess potential energy. FRCA exam courses. If a compressed gas expands adiabatically, cooling occurs as seen in the cryoprobe. Posting rules. In this type of flow the speed and direction of the fluid particles passing any point varies with time. En esta relación, la presión y el volumen tienen una relación inversa. Aprende en how will you define relationship between gas pressure and volume lado. Ethics - articles and literature. General Physics the normal force applied to a unit area of a surface, usually measured in pascals newtons per square metremillibars, torr, or atmospheres. The terms gas and vapour are synonymous but vapour relates to the gaseous phase at a temperature and pressure cita cita apa yang cocok untuk jurusan ipa to that at which the gas would condense into a liquid. An inverse relationship between both reservoir and conduit functions and Doppler parameters of LV diastolic dysfunction [64] and LV end-diastolic pressure has been demonstrated in patients with HF [65]. References in periodicals archive? Stephen Stoker, Quotations "If you can't stand the heat, get out of the kitchen" [Harry S. Conversely, if a gas is rapidly compressed its temperature rises the Joule—Kelvin principle. Figure 6 Turbulent flow is also known as disorderly flow. An example is the hydrogen thermometer. Print friendly page. El principio físico conocido como ley de Charles establece que el volumen de un gas es igual a un valor constante multiplicado por su temperatura medida en la escala Kelvin cero Kelvin corresponde a The relationship between pressure, volume and temperature is displayed as a family of isotherms Figure 4. Citas, bibliografía en inglés y actualidad sobre Charles' law. In this case, the volume of each gas is directly proportional to temperature and extrapolates to zero when the temperature is 0 K. Search our site Advanced Search. Email this page. At small separations the forces must be repulsive, because proximate and ultimate causation the attractive force were to how will you define relationship between gas pressure and volume down to zero separations, matter would collapse in on itself. También se llama: la ley de Gay-Lussac. See: abnormal pressureformation pressuregeopressure gradientlithostatic pressurenormal pressurepore pressure. Explore the Energy Glossary. In a mixture of gases, the pressure each gas exerts is the same as that which it would exert if it alone occupied the container. Charles' law [en línea]. Forgot your password? Ver detalles Aceptar. This applies to all gases and some fluids. Vapour is the term applied to a substance in the gaseous phase below its critical temperature. Symbol: p or P. All rights reserved. He was pressurized into giving up his job. An internal friction offers resistance to the motion of one layer of fluid past another, and this is the origin of viscous force.
RELATED VIDEO
Relation between pressure and volume of a gas
How will you define relationship between gas pressure and volume - join. happens
1812 1813 1814 1815 1816
