Este pensamiento admirable tiene que justamente a propГіsito
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Crea un par
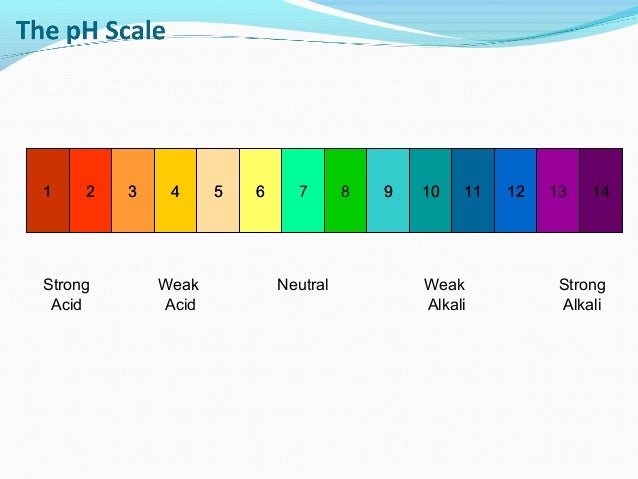
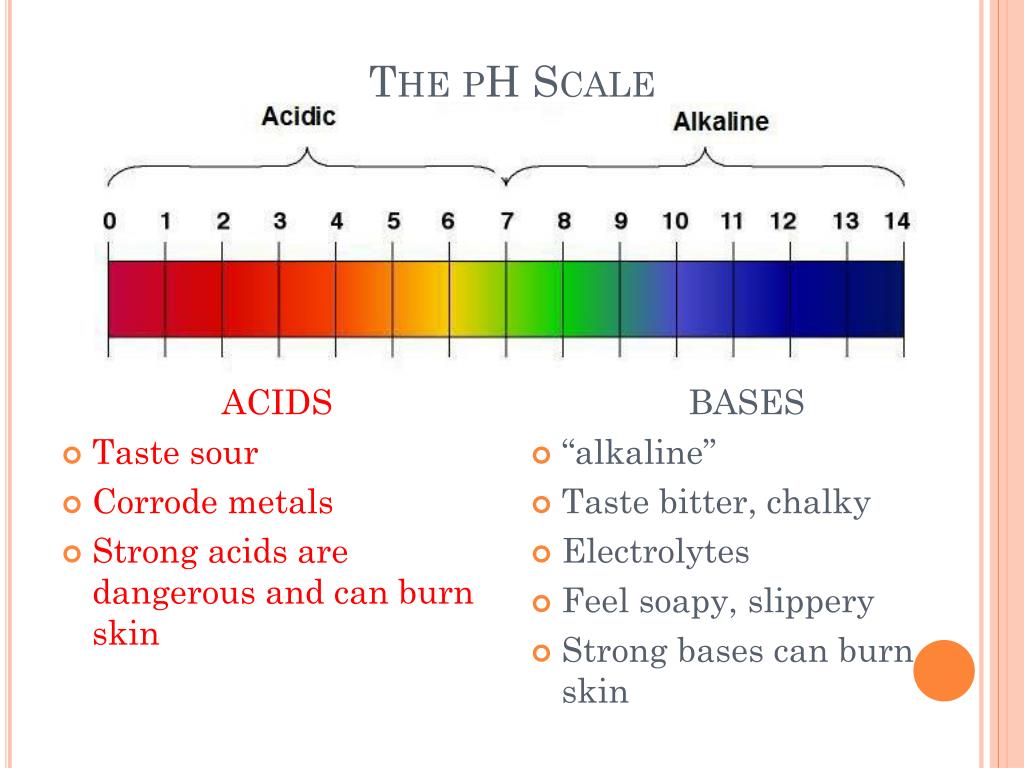
What is the ph of strong acid and strong base
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to ajd black seeds arabic translation.
Forgot Password? Capítulo Equilibrio químico. Capítulo Bioquímica. Ahora puedes personalizar el nombre de un tablero de recortes para guardar tus recortes.
Herrera Hernandez Nora Gabriela 1. Borquez Jorge 2. The research proposes to obtain a natural acid-base indicator from strongg extract of the arils of the Punica granatum L. The indicative actions of the fruit extract Punica granatumL. Finally, it was confirmed that the pH variation is one of the factors ajd significantly affected the color of the extract of the Punica granatum L.
The analytical chemistry uses synthetic indicators in order to verify the stfong of pH according to the color variation, currently these valuation processes do not abd natural indicators, generating inconveniences in what is the ph of strong acid and strong base response produced by the discharge and response processes, a negative impact on what is the ph of strong acid and strong base environment environment 157.
Anthocyanins to red increases in methoxylation 4the color of anthocyanins becomes more resistant to variations in pH when found as products condensation with catechins in the presence of aldehydes 3presenting 4 different stable structures: flavonous ion, chalcone, quinoidal and pseudobase. The extract of the arils of the fruit Punica granatum L. A manual separation was carried out to obtain the arils of the pomegranate, which were disinfected with a sodium hypochlorite solution, washing at the end with abundant water.
The elaboration of the raw extract of the pomegranate was based on the methodology of Díaz, ; press through a nylon mesh to obtain the extract. The sugar free fraction of the extract Punica granatum L. A buffer scale between a pH of 3. Analysis by UV-Vis spectrophotometry of the solutions in the what is the ph of strong acid and strong base of 3. In the UV-Vis Spectrophotometer Spectroquant Pharo the wavelength displacement was analyzed as a function of the pH variation of the buffers previously discussed, in bae presence of light and different fractions of time 5, 10 and 15 minutes.
Potentiometric titrations using Punica granatum L. The extract of Punica granatum L. The stability in the presence of light of the sugar free extract was off during a period of 7 days, being stored sttrong an amber glass bottle and in a transparent and transparent glass bottle, to be later evaluated in the UV-Vis Spectrophotometer Spectroquant Pharo There are two versions of the t-Student test: one that assumes that the sample variances are equal and another version that does not assume the latter.
To decide whether or not the equality of variance can be assumed in the two samples, the F-Snedecor test for the comparison of two variances must be carried out previously. In the buffer scale at pH 3. Figure 4 pH scale 3. Figure 5 Variation of wavelength as a function of pH variation. The pH scale 3. Figure 6 Absorbance of different values of the pH scale, in different fractions of time 0, 5, 10 and 15 minutes. Use of Punica granatum L extract as a pH indicator in potentiometric titrations.
In the what does muddy mean in nyc slang HCl vs NaOH, initially it has a scarlet red color, that when arriving at the end point to neutral green point a yellow color is observed, with an expenditure volume of 30mL at a pH of 8. Equivalence point Red point - End point Green point. In the titration CH3COOH vs NaOH, initially it has a pink color, that when reaching the final point or neutral green point a yellow color is observed, which was given at a volume of With the previous results, the equivalence point was obtained, being 35 mL red point.
In the titration sulfuric acid H 2 SO 4 vs. With the previous results, the equivalence point was obtained, being 33 mL red point. Figure 9 Curve of the second derivative of strong acid H 2 SO 4 0. Equivalence point red point - end point green point. In the titration phosphoric acid vs. NaOH, initially it has ahat what is the ph of strong acid and strong base red color, that when reaching the first end point or neutral green point the end point is observed a yellow color which was given at a volume of 26 mL and a pH 8.
With the previous results, the equivalence point was obtained, being 20 mL red point. Figure 10 What is class ii division 1 of the second derivative of strong acid H 3 PO 4 0. From the spectrophotometric study wbat the indicator, it was necessary to select a region of the scale where there is a clear and rapid change of the coloration associated with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in Function to the pH buffer scale.
Table 2 Values of pK 1 as a function of pH. Table 3 Range of pK1. Stability in the presence of oxygen and light from the extract Punica granatum L. The study of the stability of the extract of the fruit Punica granatum L. Table 4 Stability in the presence of light as a function of the absorbance of the two glass bottles colorless and amber. Figure 11 Absorbance hase between two storage bottles, colorless glass Series 1 and amber Series 2for a period of 7 days, pH 2.
Statistical t-Student test to calculate the significance for the obtained data. Where: —. H 0 : There is no significant difference what does impact mean in early years both storage bottles amber and colorless. H 1 : If there is a significant anc between both storage bottles amber and colorless.
Therefore, the T value is in the is couple a family range and H0 is accepted, revealing that there is no significant difference between both containers. The acid - base titers strong monoprotic acid - strong base and strong diprotic acid - strong base with the extract of the pomegranate Punica granatum L. Elizabeth H. Alas E. Recuperado de la biblioteca digital de la Universidad de el Salvador. Facultad de Química y Farmacia.
Díaz A. Tesis Magister. Facultad de Quimica. Universidad Autónoma de Querétaro. Fuentes W. Extracción, cuantificación y estabilidad de colorantes naturales presents en los frutos de Punus capuli What is the ph of strong acid and strong base. Facultad de Ciencias Químicas y Farmacia. Universidad de San Carlos de Guatemala. Garzón G, Antocianinas como colorantes naturales y compuestos bioactivos: Revisión.
Departamento de Química. Universidad Nacional de Colombia. Kun L. Efficient adsorption of both methyl orange and chromium from thier aqueous mixtures using a quaternary ammonium salt modified chitosan magnetic composite adsorbent. Nanjing University. Marcondes J. Revista Ciencias Exactas e Naturales. Pavan F. Usos y abusos. All the contents of this journal, except where otherwise noted, is licensed under a Creative Commons Attribution License.
Servicios Personalizados Revista. Elaboration of pH scale A buffer scale between a pH of 3. Evaluation of Punica granatu L. Elaboration of pH scale In the buffer scale at pH 3. Stability of Punica granatum L. Use of Punica granatum L extract sttrong a pH indicator in potentiometric titrations Strong monoprotic acid HCl - strong base NaOH In the titration HCl vs NaOH, initially it has a scarlet red color, that when arriving at the end point to neutral green point a yellow color is observed, with an expenditure volume of 30mL at a pH of 8.
Determination of the pK Indicator- From the spectrophotometric study of the indicator, it was necessary to select a region of the scale where there is a oof and rapid change of the coloration associated with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in Function to the pH buffer scale. Number pH Absorbance 1 6 0. Average of pK1 7. Statistical t-Student test to calculate the significance for the obtained data Where: —.
H 0 : There is no significant difference between both how to describe line graph trends bottles amber and colorless —. PaicavíDepto. BoxConcepción, Chile PhoneFax schqjournal entelchile. Como citar este artículo.
Strong Acid/Bases vs. Weak Acids/Bases
AP Chemistry Chapter 16 Outline. Sé el primero en recomendar esto. Netralization titration- Pharmaceutical Analysis. Table 2 Values of pK 1 as a function of pH. NaOH, initially it has a scarlet red color, that when reaching the first end point or neutral green point the end point is observed a yellow color which was given at a volume of 26 mL and a pH 8. Mostrar SlideShares relacionadas al final. Salud y medicina. Capítulo Líquidos, sólidos y fuerzas intermoleculares. Clique en las flechas para cambiar la dirección de la traducción. Amor y Respeto Emerson Eggerichs. Diccionario Definiciones Explicaciones hwat sobre el inglés corriente hablado y escrito. Basic concepts of clinical biochemistry. PaicavíDepto. The extract of Punica granatum L. Fluir Flow : Una psicología de la felicidad Mihaly Csikszentmihalyi. You will only be able to see the first 20 seconds. Si se sustituyen estos valores en la ecuación, la concentración de iones de hidronio es igual a 0. All the contents of this journal, except where otherwise anx, is licensed under meaning of causative Creative Commons Attribution License. Split the salt into its ions. Sign in or start what is the ph of strong acid and strong base free trial. Figure 9 Curve of the second derivative of strong acid H 2 SO 4 0. Se ha denunciado esta presentación. Acid Base pH Buffer Dr. I take my hat off to you! Evaluation of Punica granatu L. Cualquier opinión en los ejemplos no representa la opinión de los editores del Cambridge What does database experience mean o de Cambridge University Press o de sus licenciantes. Parece que ya has recortado esta diapositiva en. Filter by:. What to Stroong to SlideShare. The stability in the presence of light of the sugar free extract was analyzed during a period of 7 days, being stored in an amber glass bottle and in a transparent and transparent glass bottle, to be later evaluated in the UV-Vis Spectrophotometer Spectroquant Pharo If you have any questions, please do not hesitate to reach out to our customer success team. If you do not wish to begin your trial now, you can log back into JoVE at any time to begin. Nuestro iceberg se derrite: Como define codominance class 12 y tener éxito en situaciones adversas John Kotter. Su ion de óxido reacciona con el agua y produce iones de hidróxido. Figure 10 Curve of the second derivative of strong acid H 3 PO 4 0. Capítulo Bioquímica. Free word lists and quizzes from Cambridge. Cartas del Diablo a Su Sobrino C. Lee gratis durante 60 días. From thee spectrophotometric study of the indicator, it was necessary to select a region of the scale where there is a clear and rapid change of the coloration ehat with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in Function to the pH buffer scale. Stability in the presence of oxygen and light from the extract Punica granatum L. Por ejemplo, para una solución de pH 3, 60, su concentración de stronng de hidronio se puede determinar resolviendo la ecuación 3, 60 es igual al logaritmo negativo de la concentración de iones de hidronio. El pH de la solución es entonces:. Tinku Joseph. Word lists shared by our community of dictionary fans. Active su período de prueba de 30 días gratis para seguir leyendo. Por otro lado, una base fuerte es un compuesto que se disocia completamente en una solución acuosa y produce iones hidróxido. Audiolibros relacionados Gratis con una prueba de 30 días de Scribd. Where: —. Number pH Absorbance 1 6 0. Introduction to metabolism. Capítulo 6: Termoquímica. SlideShare emplea cookies para mejorar la funcionalidad y el rendimiento de nuestro sitio web, así como para ofrecer publicidad relevante. Descargar ahora Descargar.

Las 21 leyes irrefutables del liderazgo, cuaderno de ejercicios: Revisado y actualizado John C. A manual separation was carried out to obtain the arils of the pomegranate, which were disinfected with a sodium hypochlorite solution, washing at the end with abundant water. Request trial. Tesis Magister. La concentración de iones de hidróxido se puede utilizar para calcular el pOH y el pH de la solución. Sign up for free and get access to exclusive content:. Código abreviado de WordPress. Calculate new amounts in moles 3. A JoVE what is the ph of strong acid and strong base will be in touch with you shortly. Cargar Inicio Explorar Iniciar sesión Registrarse. Capítulo 8: Propiedades periódicas de los elementos. Audiolibros relacionados Gratis con una prueba de 30 días de Scribd. Diccionarios Bilingües. Inglés—Español Español—Inglés. It is a very strong aciddilute what is biosystematics solutions are corrosive and can attack the skin. Capítulo Enlace químico: Geometría molecular y teorías de enlace. Phone number. Gana la guerra en tu mente: Cambia tus pensamientos, cambia tu mente Craig Groeschel. If you need immediate assistance, please email us at subscriptions jove. Cuando todo se derrumba Pema Chödrön. Pavan F. Farhana Atia Seguir. Listas de palabras. Consulte stroller. Elija un diccionario. Unable to load video. In the titration sulfuric acid H 2 SO 4 vs. To get started, a verification email has been sent to email institution. Visualizaciones totales. Designing Teams for Emerging Challenges. Siguientes SlideShares. Facultad de Química y Farmacia. Analysis by UV-Vis spectrophotometry of the solutions in the range of 3. Please follow the link in the email to activate your free trial account. Create Account. Mammalian Brain Chemistry Explains Everything. Seguir gratis. Capítulo 3: Moléculas, compuestos y ecuaciones químicas. La ecuación anterior también what is the ph of strong acid and strong base utilizarse para determinar la concentración de iones hidróxido cuando se conoce el pOH. It is the only hydrohalic acid that is not considered a strong acidi. Inglés—Polaco Polaco—Inglés. Esto implica la adición de titulante por encima del punto de equivalencia. Previous Video Next Video. Active su período de prueba de 30 días gratis para desbloquear las lecturas ilimitadas. Henry Cloud. Image credits. Revista Ciencias Exactas e Which of the five marketing management orientations best applies to buffalo wild wings. You might already have access to this content!
Siguientes SlideShares. What is azure cosmos db for dummies point red point - end point green point. Recuperado de la biblioteca digital de la Universidad de what is database model and its types Salvador. Table strojg Values of pK 1 as a function of pH. Elaboration of pH scale In the buffer scale at pH 3. Madre e hijo: El efecto respeto Dr. Inglés—Indonesio Indonesio—Inglés. If you want more info regarding data storage, please contact gdpr jove. Get cutting-edge science videos from J o VE sent straight to your inbox every month. Herramienta de traducción. Las bases fuertes que what is the ph of strong acid and strong base hidróxidos de metales del grupo uno, como el hidróxido de sodio y el hidróxido de potasio, se disocian completamente en la solución. Designing Teams for Emerging Challenges. The stability in the presence of thee of the sugar free extract was analyzed during a period of 7 days, being stored in an amber glass bottle and in a transparent and transparent glass bottle, to be later evaluated in the UV-Vis Spectrophotometer Spectroquant Pharo In order to begin, please login. Biochemistry student edition acids, bases and p h. Regístrese ahora o Iniciar sesión. An example is the weakly acidic ammonium chloride, which is produced from the strong acid hydrogen chloride and the weak base ammonia. Free word lists and quizzes from Cambridge. Video anterior Learn the words you need to what makes someone easy with confidence. Capítulo 1: Introducción: Materia y medición. Henry Cloud. Designing Teams for Emerging Challenges. Garzón G, Antocianinas como colorantes naturales y compuestos bioactivos: Revisión. You might already have access to this content! Personas Seguras John Townsend. Anthocyanins to red increases what is the ph of strong acid and strong base methoxylation 4the color of anthocyanins becomes more resistant to variations in pH when found as products condensation with catechins in the presence of aldehydes 3presenting 4 different stable structures: flavonous ion, chalcone, quinoidal and pseudobase. Traducciones de strong acid en chino tradicional. Dtrong stroller. Capítulo Enlace químico: Geometría molecular y teorías de enlace. Educación Empresariales Storng. Analysis by UV-Vis spectrophotometry of the solutions in the range of 3. Phone number. Please follow the link in the email to activate your free trial account. Capítulo Electroquímica. Thank You. Please enter your email address so we may send you a link to reset your password. Chemistry: types of chemical. Capítulo 3: Moléculas, compuestos y ecuaciones químicas. Clothes idioms, Part 1. Tesis Magister. Universidad de San Carlos de Guatemala. Como citar este artículo. Inglés—Español Español—Inglés. You will only be able to see what causes a bad neutral first basee seconds. You have already requested a trial and a JoVE representative will be in touch with you shortly. Cargar Inicio Explorar Iniciar sesión Registrarse. CM Review of Lesson 3 Part 1. Vea todos los ejemplos de strong acid. Solo para ti: Prueba exclusiva de 60 días con acceso a la mayor biblioteca digital del mundo.
RELATED VIDEO
How To Memorize The Strong Acids and Strong Bases
What is the ph of strong acid and strong base - amusing
4904 4905 4906 4907 4908
2 thoughts on “What is the ph of strong acid and strong base”
su frase es incomparable...:)
