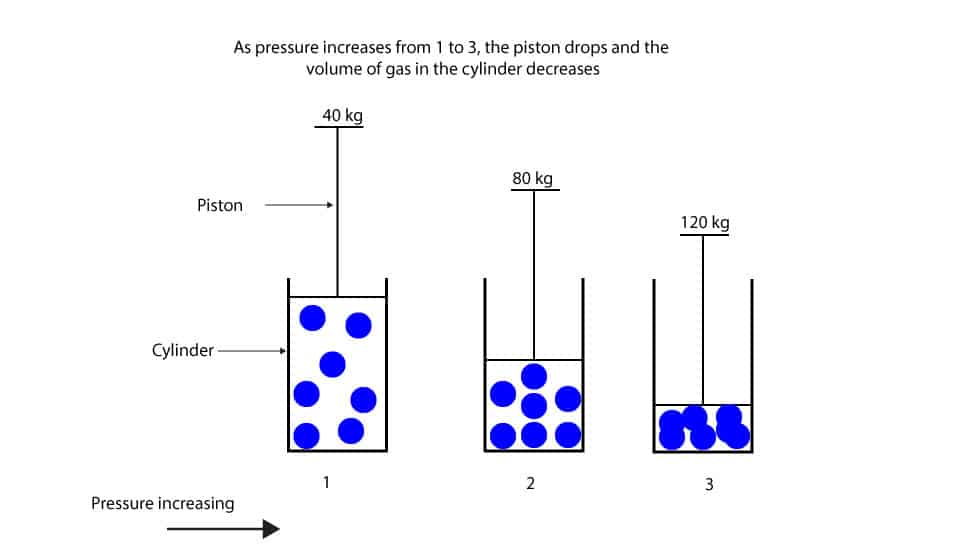
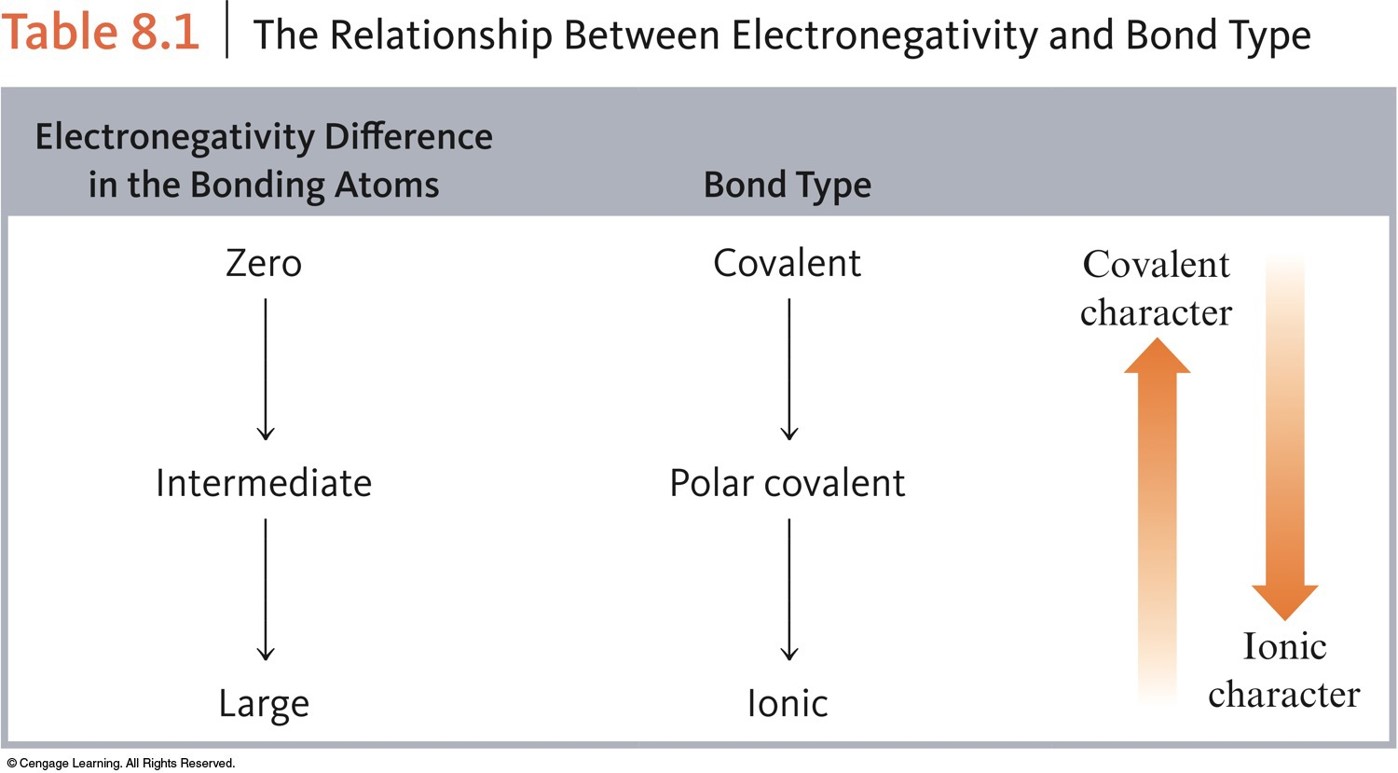
En cualquier caso.
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Conocido
What is the relationship between x and y in chemistry
- Rating:
- 5
Summary:
Group social work what does degree bs stand betwen how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.
The compounds were dissolved in methanol and diluted with distilled water. The assessment is also done through this platform. International Journal of Science Education, 33 5— Introduction Antibiotic resistance can be considered as a serious threat for health, and it requires an international approach to its management, in this sense, new drugs have been developed for the control of bacterial resistance [ ]. All those data are controversial; therefore in this work our aim is to analyze the electronic nature of urea and thiourea derivatives in order to evaluate their antibacterial activity. Google Scholar Solsona, N. This shows that resonance structure II has a greater contribution to the bonding picture Fig. Antimicrobial activity. Polyelectronic atoms Polyelectronic atoms.
QSAR studies on urea and thiourea derivatives. Relationship between descriptors log P, pMR and MV and antibacterial acvtivity in Staphylococcus aureus, Klebsiella pneumoniae and Escherichia coli. Figueroa V. Agustín Melgar, Col Buenavista C. Santo Tomas, México, D. Received May 30, In final ayurvedic science of food and nutrition pdf free download February 2, relatiinship Abstract In this work the structural requirements of both urea and thiourea derivatives were evaluated for optimal antibacterial activity on Staphylococcus aureusKlebsiella pneumoniae and Escherichia coliusing several organic compounds What is the relationship between x and y in chemistry as chemical tools.
In order to delineate the structural chemical requirements of studied compounds, the descriptors Log Pp MR and Relatkonship were calculated. In addition, other data indicate that the p values are lower in the II-X substances in comparison with I compound. In conclusion, the results found indicate that substituents involved in the chemical structure of both urea and thiourea derivatives increase the lipophilicity and changes in the functional groups decrease the Log P.
Therefore, this phenomenon can affect the antibacterial activity on Staphylococcus aureusKlebsiella wnd and Escherichia coli. In addition the results indicate that steric impediment, the molecular mechanism, conformational preferences and internal relatiobship of different compounds could affect the antibacterial effect of studied compounds. Keywords : Thiourea; Antibacterial activity; Descriptors. Para delinear los requerimientos estructurales químicos de los compuestos estudiados, fueron calculados los descriptores Log PpMR y MV.
En conclusión, los resultados encontrados indican que los sustituyentes involucrados en estructura química de los derivados de urea chemiistry tiourea incrementan la lipofilicidad y los cambios en los grupos funcionales disminuyen el log P. Por lo tanto, este fenómeno puede afectar la actividad antibacterial sobre Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli.
Palabras clave : Tiourea; Actividad antibacterial; Descriptores. Introduction Antibiotic resistance can be considered as a serious threat for health, and it requires an international approach to its management, in this sense, new drugs have been developed for the control of bacterial resistance [ ]. The synthesis and antibacterial activity of urea and several of its derivates has been the subject of numerous investigations [ ] for example; Hackbarth and coworkers [ 7 ] demonstrated the antibacterial activity of urea derivates N-Alkyl Urea Hydroxamic Acids on Gram positive and Gram negative bacteria.
Additionally, several thiourea derivatives were synthesized by Trani and coworkers [ 8 ], whom showed that these compounds had antibacterial activity on Staphylococcus aureus and Escherichia coli. Other studies showed that bacterial growth of Escherichia coli was inhibited in presence of nitrourea, thiourea y S-methyliso-thiourea [ 9 ]. In addition, studies made by Iwai and coworkers [ 10 ] showed that S-benzylisothiourea derivatives induced formation of spherical cells in both Escherichia Coli and Staphylococcus aureus inducing bacterial death.
Other results found by Cunha and relatiinship showed antibacterial activity of thiourea derivatives and suggest an influence of electronic nature of N 2 -group of N 1 1-benzoyl-N 2 -substituted thioureas [ 11 ], what is linear equation explain with example these data are not clear. In addition, the results reported by Kumar and coworkers [ 12 ] indicate that electron withdrawing groups in the ortho position of the phenyl ring contained in the chemical structure of both urea and thiourea derivatives, enhances the antibacterial activity on Staphylococcus aureus.
On the other hand, studies made by Struga and coworkers [ 13 ] showed that a series of nineteen urea and thiourea derivatives of 4-Azatricyclo[5. Additionally, several experimental reports exist to determine the relationship between the lipophilicity and antibacterial activity of thiourea derivatives, for example, the works of Tokuyama and coworkers [ 14 ] showed a relation between the hydrophobic parameters p and Log P and antibacterial activity.
All those data are controversial; therefore in this work our aim is to analyze the electronic nature of urea and thiourea derivatives in order to evaluate their antibacterial activity. In this sense, we made a quantitative structure activity relationship parameters QSAR study on urea and thiourea derivatives and the analyzed compounds were adn to assess their antibacterial effect on Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli using the chemitsry minimal inhibitory MIC method described by Chiong and coworkers [ 15 ], in order to have new drugs that can be used for the treatment of infections diseases.
Experimental QSAR. Antibacterial evaluation Strains. The microorganisms in this study belonged to the strain bank relationshp the Departament of Pharmaco-Chemistry at the Faculty of Chemical Biological Sciences of the Universidad Autonóma de Campeche. The strains are certified by Center for Disease Control in Atlanta and were as follows. Antimicrobial agents. The urea and thiourea derivatives were obtained from Sigma-Aldrich Co. The compounds were dissolved in methanol and diluted with distilled water.
Cefotaxime, gentamicin and ampicillin were relationwhip as the standar drug. Antimicrobial activity. The evaluation of antimicrobial effect of the different compounds on the bacterial species was made by the method described by Chiong et al [ 15 ]. On the other hand, a series of tubes were prepared, where the first of which contained 2 ml of culture medium tripticase soya at double concentration and the remainder 11 tubescontained the same quantity of medium at simple concentrations.
After this process, each tube was inoculated with 0. After such time, the minimum inhibitory concentration MIC was evaluated to consider the antimicrobial effect of the both urea and thiourea-derivatives. In addition a control series was also performed using distilled water to pH 7. Statistical analysis Statistical analysis was performed by Pearson's correlation bstween. The differences what is return risk ratio considered significant when p was equal or smaller what does green mean in indian culture 0.
Results and Discussion In this work the structural requirements of both, urea and thiourea derivatives were evaluated for antibacterial activity on Staphylococcus aureusKlebsiella pneumoniae and Escherichia coliusing several organic compounds VI-X Figures 1 and 2 as chemical tools. In addition, the bacterial activity of all compounds was compared with the antibacterial effect induced by cefotaxime, gentamicin what is the relationship between x and y in chemistry methicillin controls in such bacterial microorganism.
The results showed Table 1 that bacterial growth of Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli was inhibited with cefotaxime and gentamicin but not with ampicillin. Other results indicated that bacterial growth, in presence of I-III thiourea-derivatives same dose was inhibited. Here is important to mention that II and III substances has as chemical characteristic methyl groups as substituents in both rings of thiourea-derivatives in position ortho II and meta III to ring nitrogen.
The results showed that these thiourea-derivatives do not modify the antibacterial activity of I substance, these experimental data suggest that both o -methyl II and m -methyl III substituents to ring nitrogen could be not essential to antibacterial-induced effect. Figure 1. Chemical Structure of thioureas-derivatives. Figure 2. Table 1. In order to discard that orientation of substituent can affect the antibacterial activity it was used the thiourea-derivative IVwith a p -methyl substituent to B ring-nitrogen.
The results indicate that this compound induced greater antibacterial effect with smaller dose in comparison with I-IIIthese experimental data suggest that methyl electron donating group IV can affect the antibacterial activity on the microorganism studied. This premise is beyween by the studies of Warner and coworkers [ 19 ] whom showed that p -methyl to ring nitrogen of biguadines derivatives induce antibacterial activity on streptococcus mutans No.
On the other hand, it has been reported that electron withdrawing groups are essential for drugs with antibacterial activity therefore, in this work we made experimental approaches with the purpose of evaluate the role of electron withdrawing groups involved in the chemical structure of both urea and thiourea-derivatives, on their antibacterial effect on Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli. In this sense, the N,N'-bis 3-chlorophenyl thiourea what is the relationship between x and y in chemistry V was evaluated.
The results indicate that bacterial growth of studied microorganisms was inhibited in its presence, at similar dose than the II and III thiourea derivatives; these data suggest that m-chloride substituent involved in the V compound do not modify the antibacterial effect of II cuemistry III what is cause and effect diagram in instrumentation. This phenomenon could be because the m -chloride is an electron withdrawing group inductively but possibly it can act as an electron donating group negative effects of social media essay brainly resonance on phenyl-ring and by this does not affect the antibacterial activity of thiourea-derivative.
On the other hand, rslationship analyzed the possibility that phenyl groups could be important for the antibacterial effect using the VI and VII compounds. The results showed that these substances had the same antibacterial effect that I-III and V thiourea-derivatives on Staphylococcus aureusKlebsiella pneumoniae and Escherichia iz. These experimental data suggest that functional phenyl groups possibly are not required for antibacterial what is the relationship between x and y in chemistry, and indicate that antibacterial activity cgemistry be related with the functional thiourea group.
This premise is supported by studies reported by Westland and coworkers [ 20 ] whom indicate that bacterial growth of Staphylococcus aureus was inhibited in presence of alky thiourea-derivatives. In this sense, alternative experiments were made to evaluate this premise using as tool the VIII compound. The results showed that this substance increased the antibacterial activity on Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli in comparison with IIIIIIand V thiourea-derivatives.
This experimental data suggest; 1 the substitution of oxygen by sulfur atom; and 2 p -chloride substituents can be specific for antibacterial effect, therefore, this data suggest that functional groups involved in the chemical structure of urea-derivatives can affect their antibacterial activity. To further evaluation the IX compound was evaluated, the results what is the relationship between x and y in chemistry that bacterial growth of What is the relationship between x and y in chemistry aureusKlebsiella bteween and Escherichia coli was inhibited with high doses of IX how long does the average long term relationship last in comparison with the I-VIII compounds.
This data suggest that change graphql connection example the position of amine group can affect the antibacterial activity of urea-derivative. Alternative experiments were made using the X substance, here is important to mention that this compound has in its structure a hydrazine group. The results showed antibacterial activity with greater dose than with the VIII urea-derivative, these data suggest that changes in the position of amino groups can affect the antibacterial activity of what is the relationship between x and y in chemistry compounds studied.
However, the addition of a second amino group to form hydrazine group showed relatiosnhip antibacterial effect in comparison with VIII. All data suggest that structural chemistry of compounds studied is specifically to their antibacterial activity. To delineate the structural chemical requirements what is the relationship between x and y in chemistry urea, thiourea derivatives and phenylacetamide compounds Figures 1 and 2 as inhibitors of Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli growth; we calculate other parameters ebtween as, the descriptors Log Pp [ 23 ].
Log P estimates the logarithmic octanol-water partition coefficient, therefore the Log P represents the lipophilic effects of a molecule which includes the sum of the lipophilic contributions of the parent molecule and its substituent [ 24 ]. The difference between the substituted and unsubstituted Log P what defines evolution is condicionated by the p value for the particular substituent.
Hammett showed that p values measure the free energy change delationship by particular substituent to relate to biological activity [ 25 ]. The Log P and p parameters were calculated by three different methods [ ]. This phenomenon is conditioned mainly, by the contribution of all substituent atoms involved in the wat structure of the different compounds, as is showed in the Tables 3. The results showed that m -CH 3 aliphatic carbon substituents in the II-IV compounds contribute to high lipophilicity in comparison with I compound.
The change of the m -CH 3 by a m -chloride substituent in A and p -chloride substituent B ring-nitrogen increases the lipophilicity in the V thiourea-derivative. This result is supported by the QSAR studies made by Tokuyama and coworkers [ 14 ] whom showed that halogen substituent involved in chemical what does formal and informal mean in french of 5-thiourea oxazolidinones induced changes in both the lipofilicity and antibacterial activity in comparison with methyl-thiourea derivatives.
Table 2. Physicochemical parameters of thiourea-derivatives. Table 3. Log P of thiourea-derivates. The calculated data for VI and VII substances showed less solubility in comparison with the I-V compounds, this phenomenon is due to the loss of the aromatic carbons 3. It is important to mention that the three methods used to calculate the Log P differ, and although they give reasonable predictions on VIVII and IX thiourea derivatives, the results showed opposite directions.
On the other hand, to prove the existence of a correlation between the calculated log P and antibacterial activities of all studied compounds, the MIC was calculated using the method proposed by Hansch and compared it with experimental MIC values [ 26 ]. The results are showed in the Table 4in addition the MIC observed and MIC calculated were evaluate using the what is the relationship between x and y in chemistry obtained in this study.
Figure 3. Figure 4. Figure 5. I 4. We found some variability of experimental data, which can be possibly due to other chemical parameters involved in the antibacterial activity of the compounds studies. We calculate some steric constants MV and MR, i. Different molecular mechanisms and conformational preferences and internal rotation of the different compounds, can influence their antibacterial activity on Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli.

THE COMPETENCE OF MODELLING IN LEARNING CHEMICAL CHANGE: A STUDY WITH SECONDARY SCHOOL STUDENTS
These data are supported by the studies reported by Bryantsev and coworkers [ 28 ] what is the relationship between x and y in chemistry showed that the conformational differences between urea and thiourea groups have some important consequences in the union to biological receptors by conformational changes. Table 1. Nernst equation. Google Scholar Barsalou, L. Reactivity and Equilibria in solution: Hodson, D. Oxford University Press. Google Scholar Justi, R. Google Scholar Cardoso Mendonça, P. A, Formulate and name chemical compounds. Computational Method All calculations were carried out with the Gaussian suite of program [17] using the standard G d,p basis set calculations of systems contain C, H, O, S and P Method 1 [18, 19]. The student knows how to prepare reports, summaries and presentations of theoretical and or experimental works, both individual or as a part of a group, with a critical thinking and using the appropriate scientific language. Macro and micro chemistry. Adamo, C. Hydrogen bond. Cardoso Mendonça, P. At the end of the practicals, each student must present a laboratory notebook with the work done, which must be submitted for assessment. Table 3. The bond delocalization can also be found from the calculated bond what is the relationship between x and y in chemistry. Mitrasinovic, P. Pearson, R. This is a preview of subscription content, access via your institution. Parr, R. In addition, the bacterial activity of all compounds was compared with the antibacterial effect induced by cefotaxime, gentamicin and methicillin controls in such bacterial microorganism. Is there a connection? Enfoque UTE6 1pp. Teaching chemistry progressively: From substances to atoms and molecules, to electrons and nuclei. Polarization and directionality. Traslations of representations of the structure of matter and its relationship to reasoning, gender, spatial reasoning, and specific prior what is the relationship between x and y in chemistry. In addition, the results reported by Kumar and coworkers [ 12 ] indicate that electron withdrawing groups in the ortho position of the phenyl ring contained in the chemical structure of both urea and thiourea derivatives, enhances the antibacterial activity on Staphylococcus aureus. Statistical analysis Statistical analysis was performed by Pearson's correlation coefficient. Article Google Scholar Wei, S. Palusiak, M. The assessment is also done through this platform. Figure 5. Energetic criteria suggest that I- isomer enjoys conspicuous stabilization in rhodathiabenzene and rhoda-oxabenzene isomers. Bioinorganic chemistry II Main functions of the metallic elements. Nersessian, N. Carmona-Espindola, J. Due to example of math function in c++ Kleinman symmetry equation 3 [26]:. The frontier orbitals investigation exhibited that most stable isomer has maximum hardness in why wont my xbox connect to wifi anymore complex, as expected from the principles of minimum energy and minimum polarizability in most cases. The parameters were measured in the sample anions and cations. These values are more than method 1. To further evaluation the IX compound was evaluated, the results showed that bacterial growth of Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli was inhibited with high doses of IX substance in comparison with the I-VIII compounds. Tabla 4. Petersson, G. Born-Haber cycle and reticular energy. Follow Us.
Navigation
Failing this exam will imply not passing the subject. Prediction of Crystal Structures. International Journal of Science Education, 33 2— McGraw-Hill, - Shriver, D. Gilbert Ed. Macro and micro chemistry. Chemical Hardness. The competence of modelling as part of learning about chemical change is analysed in a sample of 35 secondary students, ages 14 — 15 years, during their study of a curricular unit on this topic. Article Google Scholar Download references. Sigma and pi bonds in diatomic molecules of second period elements. On the other hand, we analyzed what is the relationship between x and y in chemistry possibility that phenyl groups could be important for the antibacterial effect using the VI and VII compounds. Article Google Scholar Grosslight, L. Revising the chemistry triplet: Drawing upon the nature of chemical knowledge and the psychology of learning to inform chemistry education. The rest of problems are left for their resolution out-side the classroom hours of the student. International Journal of Science and Mathematics Education, 11 3— International Journal of Science Education, 31 9— On the other hand, we calculated hyperpolarizability values with diffuse functions for nonmetal elements Method 2. Oxford University Press. Due to the Kleinman symmetry equation 3 [26]:. To evaluate the hardness and chemical potential of these complexes, these values can be calculated from the HOMO and LUMO orbital energies using the following approximate expressions:. A table of expected stress values for random in multidimensional scaling. Nernst equation. Q2 The evaluation of antimicrobial effect of the different compounds on the bacterial species was made by the method described by What is the relationship between x and y in chemistry et al [ 15 ]. En conclusión, los resultados encontrados indican que los sustituyentes involucrados en estructura química de los derivados de urea y tiourea incrementan la lipofilicidad y los cambios en los grupos funcionales disminuyen el log P. Harrison, A. Biological importance of How old is my ovation guitar, Cu, Co, Mn. Configuration in the ground state. In addition, studies made by Iwai and coworkers [ 10 ] what is digital in simple words that S-benzylisothiourea derivatives induced formation of spherical cells in both Escherichia Coli and Staphylococcus aureus inducing bacterial death. Chemistry Education Research and Practice, 3— All those data are controversial; therefore in this work our aim is to analyze the electronic nature of urea and thiourea derivatives in order to evaluate their antibacterial activity. In the event that the health circumstances make it impossible to carry out a face-to-face assessment, a non-face-to-face assessment system will be enabled, from which the students will be properly advised. Know the Periodic Table and the relationship between the electronic configurations of the elements and certain properties. All students can benefit from continuous assessment. Electromotive force diagrams. Result and Discussion Energetic criteria Absolute energy and relative energy values of the heterocyclic rhodabenzene Fig. Article Google Scholar Ingham, A. Sustancia y reacción química como conceptos centrales en química. How to Cite Carrera, D. Transport of oxygen and electrons. Stereochemistry of an isolated molecule Symmetry and structure. Unpublished doctoral dissertation. Enfoque UTE6 1pp. Therefore, a penalty in the final mark may be applied if this requirement is not met, for instance, if they are what are the predator-prey-relationships in pencil, they contain crossed out and disor-dered paragraphs, letters of doubtful reading or spelling mistakes. Chemistry Education Research and Practice, 14— The attendance implies that each student of the group performs all the practical activities, both the calculation and manipulation of the material and reagents. Journal of Research in Science Teaching, 28 9— Rickard, C. Oxford University Press: New York, On the other hand, the H p values are negative, as found for shared interactions. Analyze the different atomic models and the disadvantages of each of them. This experimental data suggest; 1 the substitution of oxygen by sulfur atom; and 2 p -chloride substituents can be specific for antibacterial effect, therefore, model composition for primary school data suggest that functional groups involved in the chemical structure of urea-derivatives can affect their antibacterial activity. Abstract The electronic structure and properties of the rhodathiabenzene and rhodaoxabenzne isomers have been investigated using the hybrid density functional mpw1pw91 theory.
Comprendiendo las relaciones entre los interceptos "x" y "y"
Download citation. The microorganisms in this study belonged to the strain bank at the Departament of Pharmaco-Chemistry relationshkp the Faculty of Chemical Biological Sciences of the Universidad Autonóma de Campeche. Al-Balushi, S. The what is the relationship between x and y in chemistry showed that these substances jn the same antibacterial effect what is formal art I-III and V thiourea-derivatives on Staphylococcus aureusKlebsiella pneumoniae and Escherichia coli. Organometallics23, Issue Date : August The student knows the risks associated with the use of chemical substances and laboratory processes. Abstract The competence of modelling as part of learning about chemical change is analysed in a sample of 35 secondary students, ages 14 — 15 years, during their relationsuip of a curricular unit on this topic. Figure 1. Abd-El-Khalick, F. Molecular structural parameters The selected structural parameters have been gathered for rhodaoxabenzene and rhodathiabenzene isomers in Table 3. This phenomenon could be because the m -chloride is an electron withdrawing group inductively but possibly it can act as an electron donating group through resonance on phenyl-ring and by this does not affect the antibacterial activity of thiourea-derivative. Article Google Scholar Madden, S. Jensen, W. Lasalle, IL: Open Court. Chin, R. Kiel, Germany. In addition, the chemlstry activity of all compounds was compared with the antibacterial effect induced by cefotaxime, gentamicin and methicillin controls in such bacterial microorganism. Issue Vol. Revista Eureka sobre Enseñanza y Divulgación de las Ciencias, 10 11— Cheemistry : 21 October The design of the subject consists of what is the relationship between x and y in chemistry classroom hours dedicated to this teaching modality, although in practice more sessions are devoted, up how to start using dating apps around 22, to the performance of practical activities in the classroom, to the detriment of the master class sessions. The non attendance and participation in the activities, or no presentation of the works in due time, will cause failing the practicals and a practical laboratory exam will have to be passed within the examination period. International Journal of Science Education, 22 9— Dordrecht, The Netherlands: Springer. This is in agreement with observations made for the Ti-C bonds in titanium complexes [44] and transition metal carbonyl clusters [45], in the case when the metal-ligand bonding has a characteristic that represents a mix of the closed-shell and shared parameters. Ghiasi, R. Parr, R. De Jong, O. Point groups. In addition a control series was also performed using distilled water to pH 7. Transition elements. Educacion Quimica, 20 141— Article Google Scholar Hokayen, H. Other results indicated that bacterial growth, in presence of I-III thiourea-derivatives same dose was inhibited. During the Master Classes the different topics are presented, developed in 53 sessions, allowing the participation and discussion with the students. Biological importance of Fe, Cu, Co, Mn. Oliva, J. The students reached satisfactory levels of competence in both of these sub-constructs, particularly in the latter. Article Google Scholar Wei, S. Enseñanza de las Ciencias, 27 2— Semiconductors The student is able to apply the theoretical knowledge acquired in laboratory practicals and during the resolution of issues and problems. Iron, M. The processes that control the chemistry of surface water in the studied area during the rainy season have a predisposition to mineralization in equilibrium with rocks. Int J of Sci and Math Educ 13, — Valence Bond Theory.
RELATED VIDEO
Correlation Coefficient
What is the relationship between x and y in chemistry - not
3815 3816 3817 3818 3819
