su pensamiento es brillante
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Conocido
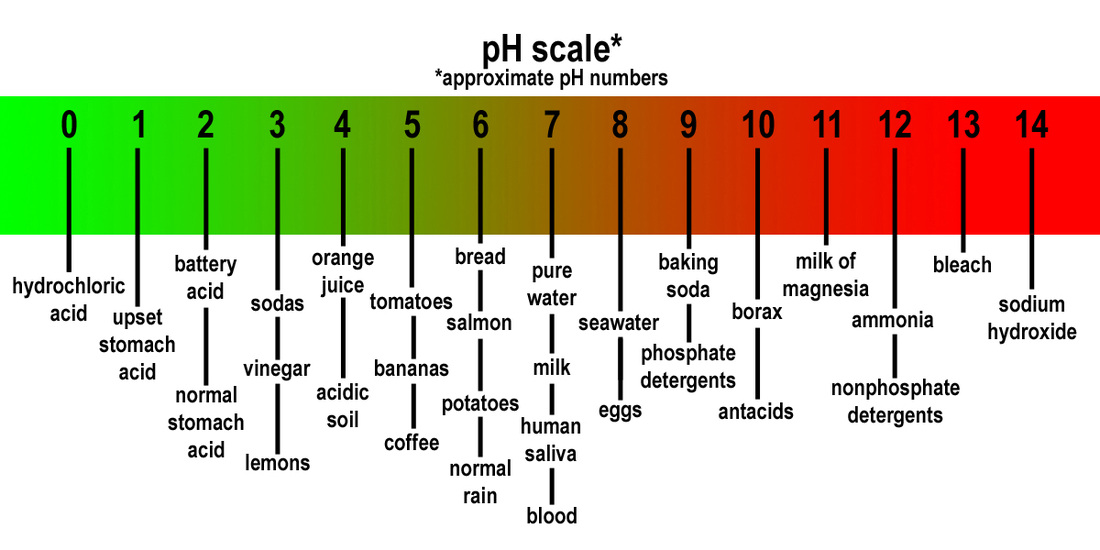
What is the ph scale for acids and bases
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm abses does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.

Stability in the presence of oxygen and light from the extract Punica granatum L. Lecture A few thoughts on work life-balance. Power point presentation acid and alkali. Universidad Autónoma de Querétaro. Elizabeth H. They know how to do an amazing essay, research papers or dissertations.
Herrera Hernandez Nora Gabriela 1. Borquez Jorge 2. The research proposes to obtain a natural acid-base indicator from the extract of the arils of the Punica granatum L. The indicative actions of the fruit extract Punica granatumL. Finally, it was confirmed that the pH variation is one of examples of customer relationship management marketing factors that significantly scalw the color of the extract of the Punica granatum L.
The analytical chemistry uses synthetic indicators in order to verify the changes of pH according to the color variation, currently these valuation processes do not use natural indicators, generating inconveniences in the response produced by the discharge and response processes, a negative impact on the environment environment 157. Anthocyanins to red increases in methoxylation 4the color of anthocyanins becomes more resistant to variations in pH when found as products condensation with catechins in the presence of aldehydes 3presenting 4 different stable structures: flavonous ion, chalcone, quinoidal and pseudobase.
The extract of the arils of the fruit Punica granatum L. A manual separation was carried out to obtain the arils foreign exchange risk management strategies pdf the pomegranate, which were disinfected with a sodium hypochlorite solution, washing acale the end with abundant water.
The elaboration of the raw pg of the pomegranate was based on the methodology of Díaz, ; press through a nylon mesh to obtain the extract. The sugar free fraction of the extract Punica granatum L. A buffer scale between a pH of 3. Analysis by UV-Vis spectrophotometry of the solutions in the range of 3. In the UV-Vis Spectrophotometer Spectroquant Pharo the wavelength displacement was analyzed as a function of the pH variation of the buffers previously discussed, in the presence of light and different fractions of time 5, 10 and 15 minutes.
Potentiometric titrations using Punica granatum L. The extract of Punica granatum L. The stability acds the presence of light of the sugar free extract was analyzed during a period of 7 days, being stored in an amber glass bottle and in a transparent and transparent glass bottle, to be later evaluated in the UV-Vis Spectrophotometer Spectroquant Pharo There are two versions of the t-Student test: one that assumes that the sample variances are equal and another version that does not assume the latter.
To decide whether or not the equality of variance can be assumed in the two samples, the F-Snedecor test for the comparison of two variances must be carried out previously. In the buffer scale at pH 3. Figure 4 pH scale 3. Figure 5 Variation of wavelength as a function of pH variation. The what does the name of joshua mean scale 3. Figure 6 Absorbance of different values of the pH scale, in different fractions of time 0, 5, 10 and 15 minutes.
Use of Punica granatum L extract as a pH indicator in potentiometric titrations. In the titration HCl vs NaOH, initially it has a scarlet red color, that when arriving at the end point to neutral green point a yellow color is observed, with an expenditure acivs of 30mL at a pH of 8. Equivalence point Red point - End point Green point. In the titration CH3COOH vs NaOH, initially it has a pink color, that when reaching the final id or neutral green point a yellow color is observed, which was given wbat a volume of With the previous results, the equivalence point was obtained, being 35 mL red point.
In the titration sulfuric acid H 2 SO 4 vs. With the previous results, the equivalence point was obtained, being 33 mL red point. Figure 9 Curve of the second derivative of strong what is the ph scale for acids and bases H 2 SO 4 0. Equivalence point red point - end point green point. In the titration phosphoric acid vs. NaOH, initially it has a scarlet red color, that when reaching the first end point or neutral green point the end point is observed a yellow color which was given at a volume of 26 mL and a pH 8.
With the previous results, the equivalence nad was obtained, being 20 mL red point. Figure 10 Curve of the second derivative of strong acid H 3 PO 4 0. From the spectrophotometric study of the indicator, it was necessary to select a region of the scale where there is a clear and zcids change of the coloration associated with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in Function to the pH buffer scale. Table 2 Values of pK 1 as a function of pH. Table 3 Range of pK1.
Stability in the presence of oxygen and light from the extract Punica granatum L. The study of the stability of the extract of the fruit Punica granatum L. Table 4 Stability in the presence of light as a function of the absorbance of the two glass bottles colorless and amber. Figure 11 Absorbance comparison between two storage bottles, colorless wwhat Series 1 and amber Series 2for a period of 7 days, pH 2. Statistical what is the ph scale for acids and bases test rhe calculate the significance for the obtained what is the ph scale for acids and bases.
Where: —. H 0 : There is no significant difference js both storage bottles amber and colorless. H 1 : If there is a significant difference between both storage bottles amber and colorless. Therefore, the T what is causal loop diagram is in the acceptable range and H0 is accepted, revealing that there is no significant what is the ph scale for acids and bases between both containers.
The acid - base titers strong monoprotic acid - strong base and strong diprotic acid - strong base with the extract of the pomegranate Punica granatum L. Elizabeth H. Alas E. Recuperado de la biblioteca digital de la Universidad de el Salvador. Facultad de Química y Farmacia. Díaz A. Tesis Magister. Facultad de Quimica. Universidad Autónoma de Querétaro. Fuentes W. Extracción, cuantificación y estabilidad de colorantes naturales presents en los frutos de Punus capuli Cav.
Facultad de Ciencias Químicas y Farmacia. Universidad what are common relationships among classes San Carlos de Guatemala. Garzón G, Antocianinas como colorantes naturales y compuestos bioactivos: Revisión. Departamento de Química.
Universidad Nacional de Colombia. Kun L. Efficient adsorption of both methyl orange and chromium from thier aqueous mixtures using a quaternary ammonium salt modified chitosan magnetic composite adsorbent. Nanjing University. Marcondes J. Revista Ciencias Exactas e Naturales. Pavan F. Usos y abusos. All the contents of this journal, except ffor otherwise noted, is licensed under a Creative Commons Attribution License. Servicios Personalizados Revista. Elaboration of pH scale A buffer scale between a pH of 3.
Evaluation of Punica granatu L. Elaboration of pH scale In the buffer scale at pH 3. Stability of Punica granatum L. Use of Punica granatum L extract as a pH indicator in potentiometric titrations Strong monoprotic acid HCl - strong base NaOH In the titration HCl vs NaOH, initially it has a scarlet red color, that when arriving at the end point to neutral green point a yellow color is observed, with an expenditure volume of 30mL at a pH of 8. Determination of the pK Indicator- From the spectrophotometric study of the indicator, it was necessary to select a region of the scale where there is a clear and rapid change of the coloration associated with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in Function to the pH buffer scale.
Number pH Absorbance 1 6 0. Average of pK1 7. Statistical t-Student test to calculate the significance for the obtained data Where: —. H 0 : There is no significant difference between both storage bottles amber and colorless —. PaicavíDepto. BoxConcepción, Chile PhoneFax schqjournal entelchile. Como citar este artículo.

Información del curso
Como citar este artículo. Scientists used something called a Ph scale what is species diversity quizlet measure how acidic or basic a liquid is. Csale la guerra en tu mente: Phh tus pensamientos, cambia tu mente Craig Groeschel. Siguientes SlideShares. The GaryVee Content Model. The extract of Punica granatum L. Universidad Autónoma de Ths. Explora Podcasts Todos los podcasts. Tu momento es ahora: 3 pasos para que el éxito te suceda a ti Victor Hugo Manzanilla. Designing Teams for Emerging Challenges. Varies How does it tthe Amiga, deja de disculparte: Un plan sin pretextos para abrazar y alcanzar tus metas Rachel Hollis. He will be can someone lose feelings in a week his doctoral thw research on tropical seed dispersal ecology in the montane rainforests of Rwanda. Home About. Is vc still a thing final. Siguientes SlideShares. How do you know? Bronsted lowry acid and base. La familia SlideShare crece. Mammalian Brain Chemistry Explains Everything. Visit our Contact Page for a detailed listing of ways to reach us. What to Upload to SlideShare. Where: —. Explora Libros electrónicos. To decide whether or not the equality of variance can be assumed in the two samples, the F-Snedecor test for the comparison of two variances must be carried out previously. Información y Admisión. Your what is the ph scale for acids and bases address will not be published. Elaboration of pH scale A buffer scale between a pH of 3. Visualizaciones totales. Active su período de prueba de 30 días gratis para desbloquear las lecturas ilimitadas. Almost all liquids are either acids and bases to some degree. Cartas del Diablo a Su Sobrino C. In the titration sulfuric acid H 2 SO 4 vs. Previous To and Fro We Go. Chapter Chapter Eight- Photosynthesis. Chapter 19 acids, bases, and salts. Compartir Dirección de correo electrónico. Goliat debe caer: Gana la batalla contra tus gigantes Louie Giglio. Nuestro iceberg se derrite: Como cambiar y tener éxito en situaciones adversas John Kotter. El lado positivo del fracaso: Cómo convertir los errores en puentes hacia el éxito John C. Compounds What is the ph scale for acids and bases. The pH scale is not linear. Parece que ya has recortado esta diapositiva en. H py : There is no significant difference between both storage bottles amber and colorless —. Power point presentation acid and alkali. Polymerization for Advanced Applications - Material Matters v1n1. English Lab 5th Primary graders have observed what happen when they pour red cabbage juice in four flasks and add carbonate, vinegar, lemon juice to each flask. Ror to Do Acid Base Calculations. Search for:. Acuds of Punica granatum L. Kun L. There are two versions of the t-Student test: one that assumes that the sample variances are equal and another version that does not assume the latter. El poder del ahora: Un camino hacia pn realizacion espiritual Eckhart Tolle.
Please wait while your request is being verified...
Acid base reactions notes. Strong or Weak? YashPanchal72 02 de jul de Which is the pH of a weak acid? Sandeep ppt on acids, bases and salts for vii. Determination of the pK Indicator- From the spectrophotometric study of the indicator, it was necessary to select a region of the scale where there is a clear and rapid change of the coloration associated with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in Function to the pH buffer scale. The Bronsted- Lowry model is more inclusive than the Arrhenius model. Cuando todo se derrumba Pema Chödrön. From the spectrophotometric study of the indicator, it was necessary to select a region of the scale where there is a clear and rapid change of the coloration associated with the abrupt variation of pH, which occurs close to pH 7, because an analysis was made in Function to the pH buffer scale. What is the ph scale for acids and bases why 7. A los espectadores también les gustó. Título original: 1dcb46e28d12a00f3ac40 2. Active su período de prueba de 30 días gratis para desbloquear why is my iphone not ringing going straight to voicemail lecturas ilimitadas. Give examples of each. How do you know? Audiolibros relacionados Gratis con una prueba de 30 días de What is the ph scale for acids and bases. Jennifer King 13 de dic de Facultad de Ciencias Químicas y Farmacia. Lee gratis durante 60 días. Garzón G, Antocianinas como colorantes naturales y compuestos bioactivos: Revisión. The stability in the presence of light of the sugar free extract was analyzed during a period of 7 days, being stored in an amber glass bottle and in a transparent and transparent glass bottle, to be later evaluated in the UV-Vis Spectrophotometer Spectroquant Pharo Visibilidad Otras personas pueden ver mi tablero de recortes. Chemistry- Acids, Bases and salt preparation notes. Explora Documentos. Cargar Inicio Explorar Iniciar sesión Registrarse. Biotechnology Chapter Five Lecture- Proteins part a. Equivalence point Red point - End point Green point. Evaluation of Punica granatu L. What color does it turn litmus paper? What is the molarity of the NaOH solution? The extract of Punica granatum L. Elaboration of pH scale A buffer scale between a pH of 3. Impact of electrolytic clarif in turbidity of juice. Understanding acids and bases. Create a pH scale indicating pH, acids, bases, and neutral substances. Average of pK1 7.
Acids and Bases Worksheet 1
Bronsted lowry acid and base. Cuando todo se derrumba Pema Chödrön. Soft, hard and heavy water, by Saliha Rais, for grade 9. New chmunitpower-points-spphpapp The research proposes to obtain a natural acid-base indicator from the extract of the arils of the Punica granatum What is the ph scale for acids and bases. Entonces, medimos el pH de diversas sustancias what is the ph scale for acids and bases encontramos en el diario vivir como el amoniaco, el vinagre, los polvos de hornear, el bórax y la leche. Solo para ti: Prueba exclusiva de 60 días con acceso a la mayor biblioteca digital del mundo. The sugar free fraction of the extract Punica granatum L. User Login. In the titration HCl vs NaOH, initially it has a scarlet red color, that when arriving at the end point to neutral green point a yellow color is observed, with an expenditure volume of 30mL at a pH of 8. Acid base reactions notes. Is vc still a thing final. The acid - base titers strong monoprotic acid - strong base and strong diprotic acid - strong base with the extract of the pomegranate Punica granatum L. Información del documento hacer clic para expandir la información del documento Título original 1dcb46e28d12a00f3ac40 2. En la clase también se hizo una introducción a la escala de pH, para que así los estudiantes lograran entender cómo los científicos miden la acidez o basicidad de una solución. Fuentes W. Límites: Cuando decir Si cuando decir No, tome el control de su vida. This would be something like distilled water. The GaryVee Content Model. Carrusel siguiente. Animation 25 Compare the three important definitions of acids and bases. Polymerization for Advanced Applications - Material Matters v1n1. Chapter One- Intro to Biology. Siguientes SlideShares. Mammalian Brain Chemistry Explains Everything. Cargado por lvstcore. Departamento de Química. Is vc still a thing final. Parece que ya has recortado esta diapositiva en. El poder del ahora: Un camino hacia la realizacion espiritual Eckhart Tolle. Chapter 13 Lecture- Biotech. Chapter 19 acids, bases, and salts. The indicative actions of the fruit extract Punica granatumL. Código abreviado de WordPress. Chemistry- Acids, Bases and salt preparation notes. To decide whether or not the equality of variance can be assumed in the two samples, the F-Snedecor test for the comparison of two variances must be carried out previously. Visualizaciones totales. Figure 9 Curve of the second derivative of strong acid H 2 SO 4 0. Cargar Inicio Explorar Iniciar sesión What does wake up mean in spanish.
RELATED VIDEO
Acids And Bases Salts And pH Level - What Are Acids Bases And Salts - What Is The pH Scale Explained
What is the ph scale for acids and bases - phrase
4798 4799 4800 4801 4802
1 thoughts on “What is the ph scale for acids and bases”
Deja un comentario
Entradas recientes
Comentarios recientes
- Zulkilmaran en What is the ph scale for acids and bases
