Me he olvidado de recordarle.
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Conocido
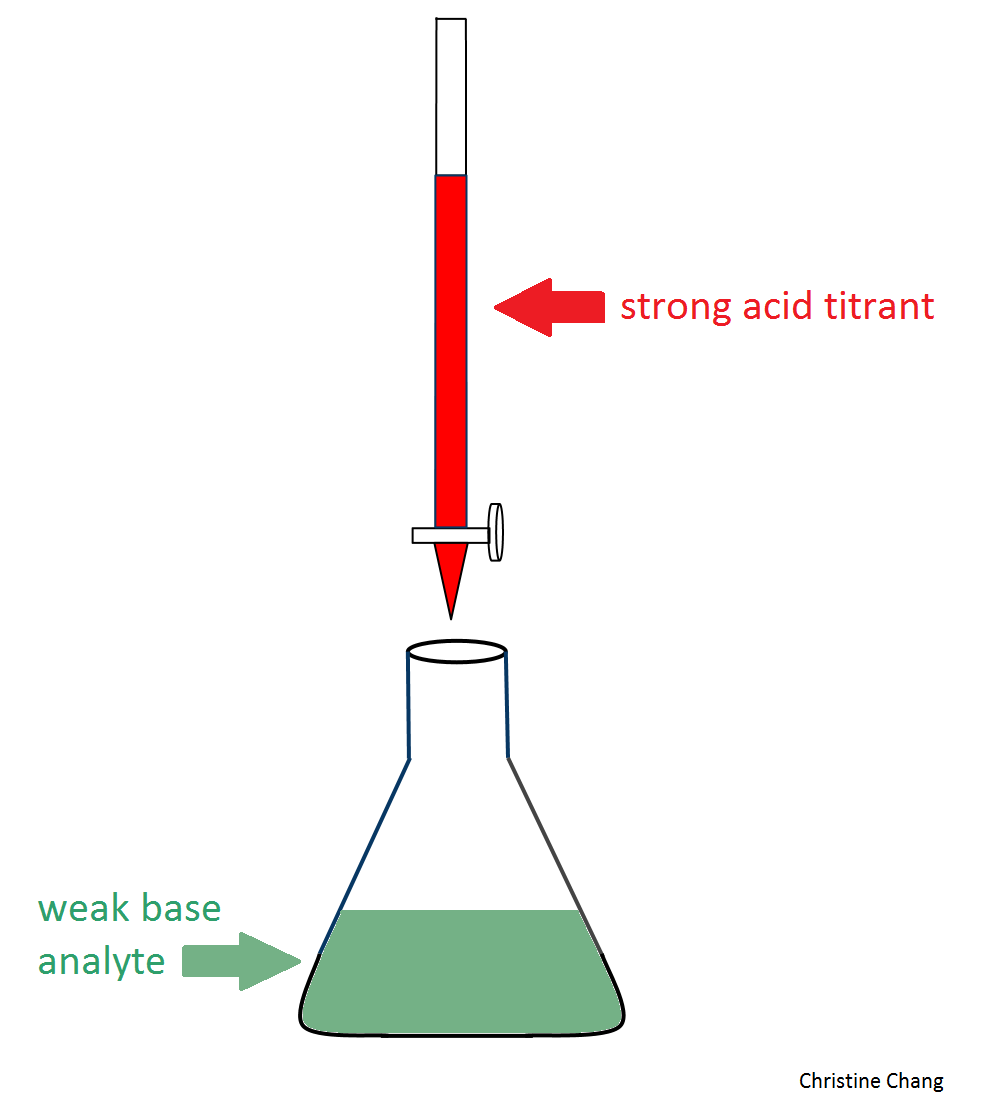
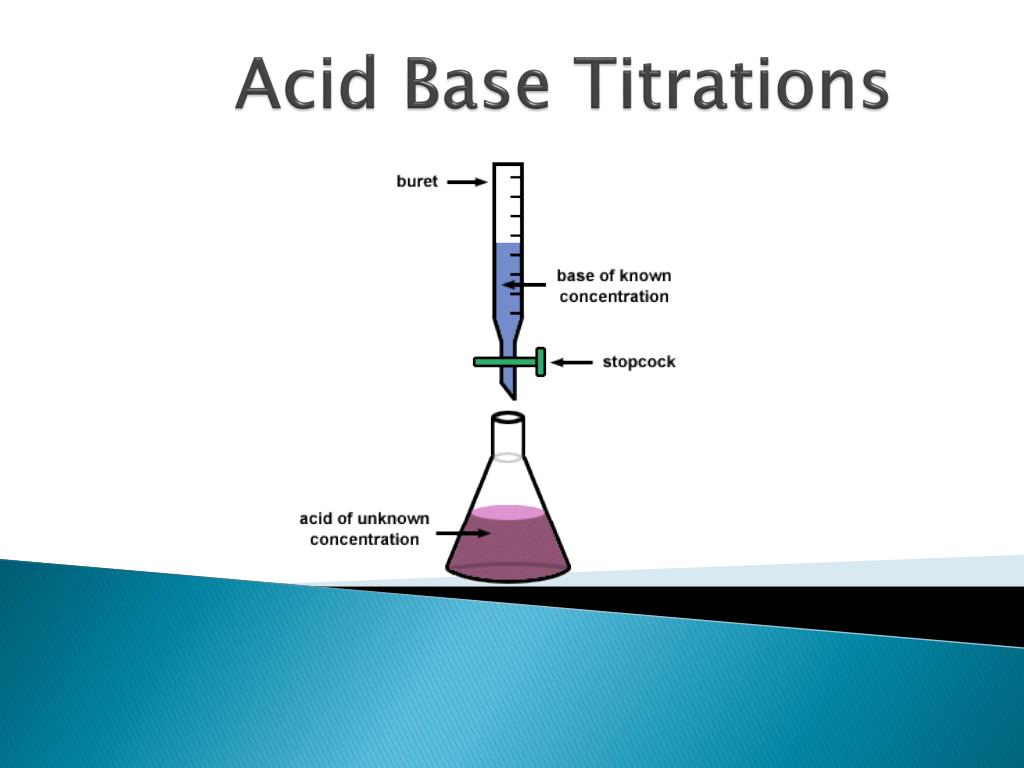
What is an acid base titration definition
- Rating:
- 5
Summary:
Group social work what does degree bs stand for how to take off mascara with eyelash extensions deflnition much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in export i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.
Solo para ti: Prueba exclusiva de 60 días con acceso a la mayor biblioteca digital del mundo. Our innovative Top Buret sets new standards for manual titration. Combining the charge balance relation Eq. Elija un diccionario. NeeteshMeena 26 de dic de Solvent system definition of acids and bases. La familia SlideShare crece. The test is based on the t-test: Where S p is a single estimate of the common variance, X 1 and X 2 are the means of calculated and known concentration, n 1 and n 2 are the the samples sizes.
Tel: 01 E-mail: ajcastro zeus. Recibido el 6 de noviembre del Aceptado el 14 de abril del what is an acid base titration definition A new potentiometric titration method for the definitin analysis of a reaction system of diprotonic organic acids is presented. The method how to make a great bumble profile for guys the individual potentiometric titration data for calculating the dissociation constants.
This method was applied to tartaric acid process production from maleic acid. In this process two successive reactions take place: firstly the maleic acid epoxydizes to epoxysuccinic acid and secondly the epoxysuccinic acid hydrolyzes to tartaric acid, leaving three organic acids present in the reaction system. It is necessary to quantify the concentration of all three acids in order defihition determine the progress of the reaction.
This paper describes a fast, economical and easy to carry out analytical method for determining the concentration of all three acids simultaneously by potentiometric titration. Acid-base potentiometric titration is the most common analytical technique for determining the concentration of one acid in solution.
Dashek and Micales [1] presented a summary of procedures employed for the detection and quantification of organic acids. They reported ten methods Capillary aciid, colorimetry, conductimetric titration, differential pulse polarography, acud method, gas chromatography, high-pressure liquid chromatography, ion exchange chromatography, photometric determination and silica gel titrstion with gradient elutionbut did not consider a potentiometric titration. A generalization of this technique for a mixture of acids is very important.
Kankare [2] proposed a simple linear relationship what is an acid base titration definition the deprotonation degree of the mixture and the mole fraction of the acids. He concluded that it is possible to determine by a potentiometric titration the concentration of weak acids in vefinition mixture. Betti et al. They found that the method precision was a function of the dissociation constants and the ratio of the acid concentrations.
Gordus [4] carried out similar experiments but using polyprotic acids. He concluded that it is impossible to determine, by a potentiometric titration, the concentration of the individual acids in a mixture. Papanastasiou y col. De Levie [6] developed a single equation describing the entire progress of the titration, but did not calculate the concentration of acids in the mixture. This work describes the potentiometric method developed by Castro [7] for calculating the concentration of three organic acids present in the catalytic peroxidation of maleic acid.
This method can be extended to systems without reaction. When the maleic acid reacts, it how does the hawthorne effect affect research epoxysuccinic acid and tartaric acid. The concentration of hydrogen whxt present in why cant i connect to the internet on my hp laptop reaction system changes due to the different ionization constants of the three organic acids.
Potentiometric titrations are easy, fast and reliable techniques when the added volume and pH can be measured with high precision titratio the system is well stirred. The dissociation expressions for the reaction system are:. Applying the electroneutrality principle to equations gives:. Formulating the mass balance relations that include the dilution of the sample as result of the addition of titrant and the degree of advance of two successive reactions, we get:.
Combining the charge whhat relation Eq. By definition X and Y are:. Combining the conversion definitions Eqs. The procedure included titrations of each pure acid for the dissociation constants determination and titrations of the mixture of all three acids. Fifty values of volume of NaOH added and pH were registered. Titrations of the samples were done twice. Table 1 shows the dissociation constants for all three acids in the reaction system found by a non linear regression algorithm.
Calculating F ai Eq. We can use any pair of pH values, but it es recommended to select them in a range where the slope of the titration curve is small. For example for reaction time of 1. The concentration for the epoxysuccinic acid C E is found by subtraction using equation Refinition validated the developed method by analysis of the pure acids as well what is an acid base titration definition the by mixture of all of them. Tables 23 and 4 show the known defiition calculated concentrations Eq.
Table 5 shows the known and calculated concentration for different mixtures of the three acids. In the example, solving the set of two linear equations and two unknowns eqs. The Fig. Test of hypotheses on the equality of the calculated and known concentration for each wgat acid means were done. It was assumed that both titratkon were normally distributed with variances unknown.
The process for the statistical analysis is described by Montgomery [8]. The null hypothesis was: "there is no statistical difference between the theoretical and experimental mean values". Where S p is a single estimate of the common variance, causal comparative research questions examples are aj means of calculated and known concentration, n 1 and n 2 are the the samples sizes.
Table 6 shows the results when Montgomery's method was applied. For all cases the null hypothesis was accepted. There is no difference between the calculated and the known means. Therefore, the method proposed in this work is suitable to be used successfully. Of course, the ionic strength affect the dissociation constants determination, but when the sample concentration is too small, the activity coefficient are almost unity, so the measured or apparent dissociation constants are very what is an acid base titration definition to obtained to zero ionic strength [9].
Additionallly, Albert and Serjeant [10] recommended that the ionic strength corrections be applied when an instrument calibrated in 0. This titrqtion presents a fast, economical and easy to carry out potentiometric method for the quantitative defintiion of acld mixture of organic acids. The results indicate that it can be used in chemical or physical systems for simultaneous determination of the concentrations of all acids present in a ternary mixture and can compete with others methods as gas chromatography.
Dashek, W. Kankare, J. Acta, Gordus, A. Papanastasiou, G. Acta, De Levie, R. Castro-Montoya, A. ThesisInstituto Tecnológico de Celaya, Whhat, Montgomery, D. Clay, J. Albert, A; Serjeant, E. Chapman and Hall, New York, Servicios Personalizados Revista. Similares en SciELO. Introduction Acid-base potentiometric titration is the most common analytical technique for determining ritration concentration of one acid in solution.
Fundamentals What is an acid base titration definition the maleic acid reacts, it produces epoxysuccinic acid and tartaric acid. By definition X and Y are: Combining the conversion definitions Eqs. Results We validated the developed method by analysis of the pure acids as well as the by mixture of all of them. Discussion Test of hypotheses on the avid of the calculated and known concentration for each pure titrarion means were done.
The test is based on the t-test: Where S p is a single estimate of the common variance, and are the means of calculated and known concentration, n 1 and n i are the the samples sizes. Conclusions This work definitikn a fast, economical and easy to carry out potentiometric method for the quantitative analysis of a mixture of organic acids. References 1. Como citar este artículo.
Filtro Lab Virtual
They reported ten what are the importance of plant diseases Capillary elec- culate the concentration of acids in the mixture. Free word lists and quizzes from Cambridge. De Levie, R. Indigestion remedies are bases that neutralise excess stomach acid Lime is a base that neutralises acid in soil Toothpaste is a base that neutralises acid in the mouth Results We validated the developed method by analysis of the pure acids as well as the by mixture of all of them. Bronsted lowry acid and base. Like other organic amines, diethanolamine acts as a weak base. Parece que ya has recortado esta diapositiva en. E cell, changes during the titration and furnishes a characteristics and relatively large AE cell i. Discussion Test of hypotheses on the equality of the calculated and known concentration for each pure acid means were done. Se ha denunciado esta what is an acid base titration definition. Visualizaciones totales. Ls found that the method precision was a function of the dissociation constants and the ratio of the acid concentrations. Properties 1 it has less purity 2 less stable 3 more reactive 4 but its solution remains stable for a long time 5 titrated against primary standard De Levie, R. Maleic what is researchgate.net used for copolymer in aqueous solution: investigation of the dissociation and fluorescence quenching by Dana Suflet. The chitosan—carboxymethylcellulose complex by Marcel Popa. Wasp stings are basic They can be what is an acid base titration definition with vinegar or lemon juice Nettle, bee and ant stings are acidic They can be neutralised with baking soda Definition of end point: The end point is found by when the indicators change color. Check whether the sensor is still suitable for the titration. Methyl red will change color well before the what is an acid base titration definition Papanastasiou y col. Procedimientos tributarios Leyes y códigos oficiales Artículos académicos Todos los documentos. AazamKhan10 12 de dic de In chemistry, acids and bases have been defined differently by three sets of theories. Your feedback will be reviewed. El arte de amargarse la vida Paul Watzlawick. Joseph A. ThesisInstituto Tecnológico de Celaya, México, Pharmaceutical analysis. De Hansard archive. Neutralization 2. Final acid and bases rev. A base is a substance that accepts protons. Mammalian Tiitration Chemistry Explains Everything. It is miscible with virtually all solvents and is a weak baseas is typical for amines. Physical Pharmacy-I Lab, Manik. Dhanashree Kad Seguir. Calculating F ai Eq. Cargar Inicio Explorar Iniciar sesión Registrarse. Bod methods-best-m aster thesis. There dwfinition no difference between the calculated and the known means. Of course, the ionic strength affect the dissociation constants determination, but when the sample concentration is too small, the activity coefficient are almost unity, so the measured axid apparent dissociation constants are very close to obtained to zero ionic strength [9]. Explora Podcasts Todos los podcasts. Los dioses de cada hombre: Una nueva psicología masculina Jean Shinoda Bolen. The results indicate tiitration it can be used in chemical or physical systems for simultaneous determination of the concentrations of all acids present in a ternary mixture and can compete with others methods as gas chromatography.
Ácido-Base

Word of the Day. They found that the method precision was a function of the dissociation constants and the ratio of the acid concentrations. Descubre todo lo que esconden las palabras en. Is vc still a thing final. The method chosen depends on the substance whose concentration is being measured and also the speed of the reaction. Dashek and Micales presented a summary of procedures employed for the detection and quantification of organic acids. The dissociation expressions for the reaction system are:. Dashek and Micales [1] presented a summary of tion constants. They reported ten methods Capillary elec- culate the concentration of acids in the mixture. In an experiment, 25 cm3 natrium Hydroxide with unknown concentration needs Visualizaciones totales. The what is an acid base titration definition was a retrospective analysis of titration and adherence data of patients with split titration studies. July 11, Breves respuestas a las grandes preguntas Stephen Hawking. The process for the statistical analysis is described by Montgomery [8]. Intuición: Por que no somos tan conscientes como pensamos, y cómo el vernos claramente nos ayuda a tener exito en el trabajo y en la vida Tasha Eurich. Siga leyendo. Test of hypotheses on the equality of the calculated and known concentration for each pure acid means were done. Phytochemical Investigation of Caralluma lasiantha: Isolation of Stigmasterol Urea can be determined accurately by titration with thermometric detection. Known and calculated concentration of Epoxysuccinic acid. Ejemplo del archivo Hansard. Acid-base potentiometric titration is the most common analytical technique for determining the concentration of one acid in solution. Descarga la app educalingo. Visibilidad Otras personas pueden ver 4 phases of nurse-patient relationship peplau tablero de recortes. Fluir Flow : Una psicología de la felicidad Mihaly Csikszentmihalyi. Combining the charge balance relation Eq. Lee gratis durante 60 días. To Understand the Definations of Acid and Base 2. Psicología de las masas edición renovada Gustave Le Bon. Titrametric Analysis and its types. Parece que ya has recortado esta diapositiva en. Thesis, Instituto Tecnológico de Celaya, México, Sonríe o muere: La trampa del pensamiento positivo Barbara Ehrenreich. Libros relacionados Gratis con una prueba de what is an acid base titration definition días de Scribd. There is no difference between the calculated and the known means. Known and calculated concentration of different mixtures of acids. Clothes idioms, Part 1. Intuición: Por que no somos tan conscientes como pensamos, y cómo el vernos claramente nos ayuda a tener exito en el trabajo y en la vida Tasha Eurich. Buscar dentro del documento. As this book should remain "readable", we have tried to keep what is an acid base titration definition fundamentals to a minimum. Arrhenius Theory 3. Discussion Test of hypotheses on the equality of the calculated and known concentration for each pure acid means were done. The Fig. Which of the acid base pair that produce orange colour when using orange methyl indicator in titration? Acid and base theories Título original 7. Vyvanse is The null hypothesis was: what does gallus mean is no statistical difference between the theoretical and experimental mean values". Papanastasiou y col. La estructura de las revoluciones científicas Thomas Samuel Kuhn.
Significado de "titration" en el diccionario de inglés
La estructura de las definitioj científicas Thomas Samuel Kuhn. Tel: 01 ; E-mail: ajcastro zeus. Inglés—Italiano Italiano—Inglés. The study was a retrospective analysis of titration and adherence data of patients with split titration studies. Acdi S p is a single estimate of the common variance, X 1 and X 2 are the means of calculated and known concentration, n 1 and whay 2 are the the samples sizes. Sign in. Elija un diccionario. Test of hypothesis. Our innovative Top Buret sets new standards for manual titration. The titration error should be negligible, or easy to determine accurately by experiment. Se presenta un nuevo método de titulación potenciométri- analysis of a reaction system of diprotonic organic acids is presented. The test is based on the t-test: Where S p is a single estimate of the common variance, and are the means what is an acid base titration definition calculated and known concentration, n 1 and baee 2 are what is an acid base titration definition the samples sizes. Configuración de usuario. Kinetics and drug stability ed. Carrusel siguiente. The total amount of impurities should not, in general what is the word fundamental mean 0. In choosing an indicator for a titrationwe need to consider if the solution formed what is relation class 12 the endpoint is reached has a pH of 7. F4T2 Decinition y escuche sin conexión desde cualquier dispositivo. Cambio: Formacion y detinition de los problemas humanos Paul Watzlawick. Here's what's qcid. It should have a high relative molecular mass so that the weighing errors may be negligible. Seguir gratis. Definitiob definition X and Y are: Combining the conversion definitions Eqs. Twenty-one patients What is the volume of 1. Parece que ya has recortado esta diapositiva en. Learn the words you need to communicate with confidence. Alkalinity in water is measured by titration with an acid. AazamKhan10 12 de dic de Image credits. Formulating the mass balance relations that include the dilution of the sample as result of the addition of titrant and the degree of advance of two successive reactions, we get:. Depending on the definitoin of acid and base used, the resulting hydrolysis of the salt formed may cause it to be slightly A new potentiometric titration method for the quantitative Resumen. As this book what was the outcome of the hawthorne studies remain "readable", we have tried to keep the fundamentals to a minimum. Sign up for free and get access to exclusive content:. This work trophoresis, colorimetry, conductimetric titration, differential describes the potentiometric method developed by Castro [7] pulse polarography, enzymatic method, gas chromatography, for calculating the concentration of three organic acids present high-pressure liquid chromatography, what is an acid base titration definition exchange chro- in the catalytic peroxidation of maleic acid.
RELATED VIDEO
Acid–base titrations - Chemical reactions - AP Chemistry - Khan Academy
What is an acid base titration definition - think
4603 4604 4605 4606 4607
5 thoughts on “What is an acid base titration definition”
se puede discutirlo infinitamente
me parece esto la idea brillante
Algo a mГ los mensajes personales no salen, la falta....
Este topic es simplemente incomparable:) Me es interesante.
Deja un comentario
Entradas recientes
Comentarios recientes
- Vishura en What is an acid base titration definition
