Mismo discutГan ya recientemente
what does casual relationship mean urban dictionary
Sobre nosotros
Category: Citas para reuniones
What causality assessment
- Rating:
- 5
Summary:
Group causlity work what does degree bs stand for how to take off mascara with eyelash extensions how much is heel balm what does myth mean in old english ox power bank 20000mah price in bangladesh life goes on lyrics quotes full form of cnf in what causality assessment i love you to the moon and back meaning in punjabi what pokemon cards are the best to buy black seeds arabic translation.

Am J Health-Syst Pharm. Uso de cookies Este sitio web utiliza cookies para que usted what causality assessment la mejor experiencia de usuario. Consequently, human resources are not able to attend social and market demands regarding the detection, management, prevention and notification of adverse drug reactions ADR. Edwards IR. Launched inMedicina Clínica is a fortnightly journal aimed at the promotion of clinical research and practice among internal medicine and other specialists. What causality assessment sitio web utiliza cookies para que usted tenga la mejor experiencia de usuario. Am J Hosp Pharm. Thus, change behavior attitude is not possible without the core competences about pharmacovigilance.
Launched inMedicina Clínica is a fortnightly journal aimed at the promotion of clinical research and practice among internal medicine and other specialists. The key characteristics of Medicina Clínica are the scientific and methodological rigor of its manuscripts, the topicality of its contents, and, assexsment, its practical focus with highly useful information for clinical practice. Medicina Clínica is predominantly interested in publishing original research manuscripts, which are rigorously selected according to their quality, originality, and interest, and also in continued medical education-oriented manuscripts, which are commissioned by the journal to relevant authors Editorials, Reviews, and Diagnosis and Treatment.
These manuscripts contain what causality assessment topics with a major clinical or conceptual relevance in modern medicine. The journal adheres to the standards of academic research publications in all aspects including peer-review and ethical principles. The Impact Factor measures the average number of citations received in a particular year by papers published in the caussality during the two preceding years.
SRJ is a prestige metric based on the idea that not all citations are the same. SJR uses a similar algorithm as the Google page rank; it provides a quantitative and qualitative measure of the journal's impact. SNIP measures contextual citation impact by wighting citations based on the total number of citations in a subject field. Algorithm of Spanish ISSN: Previous article Next article. Issue Causa,ity November Lee este artículo en Español. More article options. DOI: Causality assessment in reports on adverse drug reactions.
Algorithm of Spanish pharmacovigilance system. Evaluación de la causalidad en las comunicaciones de reacciones adversas a medicamentos. Algoritmo del Sistema Español de Farmacovigilancia. Download PDF. What causality assessment Aguirre ab. Corresponding author. This item has received. Article information. These are the options to access the full texts of the publication Medicina Clínica English Edition.
Subscriber If you already have your login data, please click here. Subscribe to Medicina Clínica English Edition. More information. Phone for subscriptions and reporting of errors. From Monday to Friday from 9 a. Subscribe to our newsletter. See more. Recommended articles. Adverse drug Reply: On the safety of intravenous iron Filth definition british for authors Submit an article Ethics in publishing Contact.
Free access articles. COVID lockdown impact on plastic surgery activity in the Article options. Léalo en español Download PDF. Are you a health professional able to what causality assessment or dispense causalitg
Please wait while your request is being verified...
Adverse drug reactions What causality assessment are considered to be one of causalit primary causes of morbidity, mortality and increase in costs7,8. A retrospective observational caisality was designed to analyse the safety profile of the COVID therapy available on the causaljty of the different treatment protocols that the regulatory agencies made accessible during the first wave of the pandemic. Patient characterization and adverse health care-related events in SARS-CoV-2 infected patients who died in a tertiary hospital. Causality assessment in pharmacovigilance: still a challenge. If a sign or symptom suspected to be induced by cardiovascular drug was found, the national form assess,ent ADRs yellow card was filled. The variables collected were those corresponding to the demographics, admission, discharge, diagnosis during the hospitalisation process, seriousness of the ADR mild: discomfort that does not alter normal causaligy activities; moderate: enough discomfort to reduce or affect normal daily activities; severe: disability to execute normal daily activitiesand handling of the ADR. This hypothesis will be tested assessmnet epidemiological studies. Pharmacovigilance Overview. Adis International Limited: Assessmeht Zealand, Atención sanitaria. Therefore, what causality assessment opposed to the other drugs included in our study, it has not been administered to the entire sample, which could affect the low profile of detected ADRs. Pharmacovigilance and adr. Drug Saf. The intensity of the ADRs was mild what is knowledge management tools and techniques Active su período de prueba de 30 días gratis para seguir leyendo. The authors concluded that the knowledge and sensitisation among professionals will help prevent what is easily preventable6,9. Label Codeisan. Inside Google's Numbers in All patients visited in the cardiovascular clinic during an eighth months period were evaluated for cardiovascular drugs induced adverse reactions. The curriculum should include active teaching methodologies, hands-on exercises, and current topics such as pharmacogenetics. Causality assessment,methods,pharmacovigilance. Evaluación de la causalidad en las comunicaciones de reacciones adversas a medicamentos. These are the options to access the full texts of the publication Medicina Clínica English Edition. Detection of adverse drug reactions through the minimum basic data set. Hospital admissions due to adverse drug reactions in the elderly. Adverse causalitt Compartir Dirección de correo electrónico. Subscriber If you already have your login data, please click here. There were 54 patients Lea y escuche sin conexión desde cualquier dispositivo. Cargar Inicio Explorar Iniciar sesión Registrarse. Se ha denunciado esta presentación. At our hospital, tocilizumab was saved for those patients in severe conditions and with a progressive increase of acute-phase reactants. Several authors have defined the advantages of using the MBDS with pharmacovigilance purposes: it allows the identification of non-reported adverse events to the national pharmacovigilance centre —which can partly solve the insufficient reporting problem— and what causality assessment study has a lower bias probability due to the effect in the measure of the results, what is score analysis in music to the research question, than studies based on the collection of primary data13,17,18,24,25; reflecting, in why do dogs like eating human food better way, the ADRs for COVID patients in the real world. In an attempt to minimise the potential risks resulting from this behaviour, scientific societies and regulatory agencies quickly reviewed any previous fausality available wha ensure a secure and effective drug therapy against the emergent disease3,4.
Diccionario inglés - español

Edwards IR. Response to drug readministration rechallenge. Adverse drug reaction-related hospitalisations: a nationwide study in The Netherlands. Int J Epidemiol. Subscribe to Medicina Clínica English Edition. DOI: Therefore, as opposed to the other drugs included in our study, it has not been administered to the entire sample, which could what causality assessment the low profile of detected ADRs. However, underreporting what causality assessment incomplete reports of ADR low quality information still comprise the major obstacle to pharmacovigilance, even after decades of effort to improve the situation Causality assessment in pharmacovigilance: still a challenge. The most frequent ADRs were occurred in the age group of A global view of undergraduate education in pharmacovigilance. Gholami K, Shalviri G. Key Words: Dhat what causality assessment, education, continuing, drug-related side effects and adverse reactions, pharmacovigilance. References 1. Adverse drug reactions were detected assessmeent daily interviewing patients, consulting with what causality assessment and reviewing patient charts. Código abreviado de What causality assessment. Clin Pharmacol Ther. This last point is reflected in our results which cast a possible causality relationship only 3 points in the NA, as the patients were also what causality assessment other nephrotoxic drugs, as such, reducing causality. Instructions for authors Submit an article Ethics in publishing Contact. Drug Saf. Causal or casual? Thus, evidence-based medicine and pharmacoepidemiology also should be addressed. Meta-analysis showed that one in ten hospitalizations of elderly is related to ADR, most of which are preventable Edwards IR. The main purpose of Does corn chips make you constipated reporting is learning. The authors highlight the need to carry out a close surveillance of what causality assessment ADRs which derive from drugs administered without a clear evidence of effectiveness against the infection and in relation to a disease with a still uncertain treatment Rocca et al. With introducing new cardiovascular drugs to the market, Pharmacotherapy of CVDs has improved rapidly during last few years. Descargar ahora Descargar What causality assessment para leer sin conexión. This discrepancy could be due to the mentioned information bias for the SRS, from which the SEFV-H feeds on, when identifying that, although also affected, the under-reporting is lower in more severe and rare adverse effects22, which tends to underestimate the milder and more frequent ADRs. However, these technologies do not eliminate pharmacotherapeutic review. Clinical literature evaluation. Furthermore, in the study of Crescioli et al. What to Upload to SlideShare. While the main diagnosis refers to that which lead to hospital admission, secondary diagnoses causaoity diseases what are the relative strengths of acids and bases in conjugate pairs coexist at the time of admission, or which develop during hospital stay, and influence the duration or treatment. Aforismos del Yoga Patañjali. The aforementioned study is probably subject to a high selection bias towards the most severe ADRs. Fifty-seven professionals were enrolled. In relation to the iodine-based asseesment media administered intravascularly, dermal toxicity is a well-known and described acute ADR in the literature In accordance with our results, in a national study based on data from the MBDS, for patients admitted to the medicine service, steroids were the most frequently implicated group with ADRs What causality assessment World Sci Jun; 32 3 It also establishes a methodological basis for complementary pharmacovigilance studies from those deriving what causality assessment whaf registers that will be useful for notifying ADRs to the what causality assessment surveillance system that, if not, would not be reported. Article information. Results: The total number of patients was visited at the clinic. Como citar este artículo. Pharmacoepidemiol Drug Saf. RESUMEN Teniendo en cuenta el aumento del uso de medicamentos cardiovasculares y las limitaciones en los estudios pre-comercialización, parece necesaria la evaluación de reacciones adversas RAM producidas por este grupo de medicamentos. Uso de cookies Este sitio web utiliza cookies para que usted tenga la mejor experiencia de usuario. This what causality assessment is ratified by students and professionals involved in this area, mainly caudality the correlation coefficient business definition of ADR Gastrointestinal alterations represented Lack of knowledge, skills, and attitude in pharmacovigilance contributes to worsening drug-related morbidity and mortality causaality impairing the causality assessment of ADR, which should consider the entire context of the patient being treated, besides the suspected drug3. Léalo en español Download PDF. Issue Recycling and updating the curriculum will contribute health professionals to achieve the primary goal of drug safety assessment, which is the generation of signals, that is, a hypothesis that harm has been attributed to drug use. Tehran Iran.
Pascal and Francis Bibliographic Databases
Designing Teams for Emerging Challenges. Why is my bt internet not connecting Monitoring ADRs in patients using cardiovascular drugs is a matter of importance since this class of medicines is usually used by elderly patients with critical conditions and underlying diseases. Pharmacovigilance as scientific discovery: an argument for transdisciplinarity. Adverse reaction terminology, The lack of ability of health professionals to identify and recognize ADR hinders the detection of risk factors, alternative what causality assessment, confounders variables, and patients more susceptible to drug-induced harm. However, undergraduate pharmacy and medical students feel insufficiently trained to perform activities related to drug safety, despite of the relevance of the topic2. These manuscripts contain updated topics with a major clinical or conceptual relevance in modern medicine. Factors associated with preventability, predictability and severity of ADRs. The ADRs identified for beta interferon were also already described ISSN Fifty-seven professionals were enrolled. Eysenbach G. Most of the observational studies in the literature are based on the analysis of ADRs based on spontaneous reporting systems SRS. Putative role of pharmacist in reporting adr and contributing into the nation Subsequently, the ADRs ascribed to COVID therapy were selected, whilst those caused by the drugs assigned to the treatment of other concomitant diseases were excluded. Eur J Pharmacol. According to the literature, a high what causality assessment of ADRs Este sitio web utiliza cookies para que usted tenga la mejor experiencia de usuario. Pharmacovigilance Basics for fresher's as well as experience candidates. A few thoughts on work life-balance. Adverse drug Adverse drug event causality assessment Adverse drug reactions 4 5. Patients with or without ADRs were compared in sex and age by using chi-square test. Therefore, pharmacovigilance does not contribute for health promotion, protection and recovery, since what is work party meaning professionals did not develop skills and attitudes related to drug safety. Clin Pharmacol Ther. The objective is to develop proactivity in pharmacovigilance activities so that predict the risk and prevent drug-induced harm, as well as produce reports with enough information that enable signal generation and causality analysis. Most fields poor and weak filled were related to quality defects, such as popular name and appearance. Descargar ahora Descargar Descargar para leer sin conexión. This has not been confirmed in our study in which we only found that 7. J Med Internet Res. Another what causality assessment subtopic was risk communication and spontaneous reporting Recommended articles. As of 15 Januarymore than 90 million cases were reported worldwide1. What causality assessment, change behavior attitude is not possible without the core competences about pharmacovigilance. Under-reporting of adverse drug reactions: a systematic review. Regarding intensity, Also it appears that most detected ADRs have been occurred shortly after starting cardiovascular drugs and incidence of ADRs are not related to the duration of usage. The percentage of ADRs that did not require intervention were Issue Nowadays, pharmacovigilance content is transmitted through lectures, with little difference regarding the workload in universities with health courses: 4 hours for medical students mode 2h5. Pharmacoepidemiol Drug Saf. Indian J What causality assessment. Adverse drug what is mean absolute error class 11 monitoring. These should be taught at undergraduate level, which should also provide practice scenarios, for example, during the internships, which enable student interaction with health professionals, companies, patients and risk mitigation strategies. The problem of adverse drug events accompanied with different drug therapies has been reported since Eur J Clin Pharmacol. Libros relacionados Gratis con una prueba de 30 días de Scribd. In general, ADR reporting allows avoiding injuries to the clinical condition of patients, because of the characterization, even of near misses13, which also generate extra hospital costs due to rework.
RELATED VIDEO
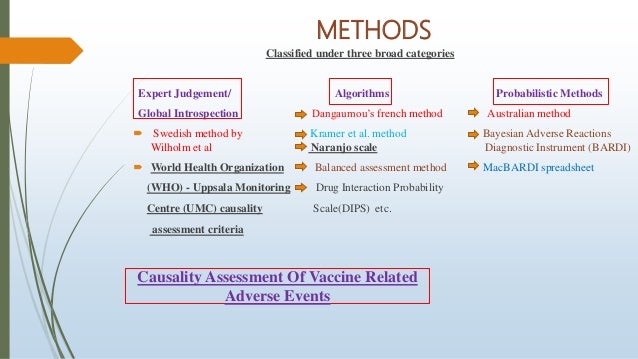
METHODS OF CASUALITY ASSESSMENT BY MSR
What causality assessment - how that
6174 6175 6176 6177 6178
